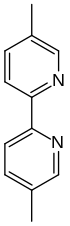
Abametapir
 | |
| Names | |
|---|---|
| Trade names | Xeglyze |
| Other names | Ha44 |
IUPAC name
| |
| Clinical data | |
| Drug class | Pediculicide |
| Main uses | Head lice infestation[1] |
| Side effects | Skin redness, rash, burning, vomiting, eye irritation, itching, hair color changes[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Topical |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 91.3–92.3% |
| Metabolism | CYP1A2 |
| Metabolites | Hydroxyl and carboxyl derivatives |
| Elimination half-life | 21 hours |
| Chemical and physical data | |
| Formula | C12H12N2 |
| Molar mass | 184.242 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Abametapir, sold under the brand name Xeglyze, is a medication used to treat head lice infestation.[1] It can be used in people at least six months old.[1] It is applied to the hair and scalp.[1]
Common side effects include skin redness, rash, burning, vomiting, eye irritation, itching, and hair color changes.[1] If swallowed, it can result in benzyl alcohol toxicity.[1] While there is no evidence of harm in pregnancy, such use has not been well studied.[1] It is a metalloproteinase inhibitor.[1]
Abametapir was approved for medical use in the United States in 2020.[1] It is not approved in either the United Kingdom or Europe as of 2022.[3] It is not commercially available in the United States as of 2022.[4][3]
Medical uses
Abametapir is indicated for the topical treatment of head lice infestation in people six months of age and older.[2][5]
Dosage
It is applied as a single treatment.[4]
Contraindications
Abametapir has no contraindications according to the labeling.[6]
Side effects
Common side effects are burning skin sensations (in 3% of patients), contact dermatitis (2%), skin redness (4%), rash (3%), and vomiting (2%).[6]
Interactions
Abametapir blocks the liver enzymes CYP3A4, CYP2B6 and CYP1A2 in vitro. A single application of the drug may lead to increased blood concentrations of drugs that are metabolized by these enzymes.[2]
Pharmacology
Mechanism of action
The drug inhibits enzymes called metalloproteinases. In lice, these enzymes play a role in egg development and survival;[2] and consequently, blocking them will disrupt the lice's life cycle.
Pharmacokinetics
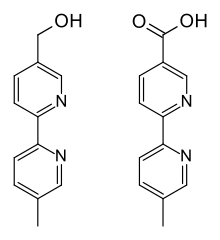
After application to the scalp, part of the substance reaches the bloodstream, where most of it (91.3–92.3%) is bound to plasma proteins. It is metabolized primarily by the liver enzyme CYP1A2 to abametapir hydroxyl and further to abametapir carboxyl (see structure drawings). Abametapir carboxyl has a plasma protein binding of 96.0–97.5% and is the predominant of the three substances in the circulation, having a Cmax 30 times and an area under the curve (AUC) 250 times that of abametapir itself.[2]
The elimination half-life of abametapir is 21 hours. That of abametapir carboxyl is not well known; it is thought to be 71±40 hours or longer. It is not known whether the drug is eliminated via the urine or the faeces.[2]
History
The U.S. Food and Drug Administration (FDA) approved abametapir based on evidence from two identical clinical trials of 699 participants with head lice.[5] The trials were conducted at fourteen sites in the United States.[5]
The benefit and side effects of abametapir were evaluated in two clinical trials that enrolled participants with head lice who were at least six months old.[5]
About half of all enrolled participants was randomly assigned to abametapir and the other half to placebo.[5] Abametapir lotion or placebo lotion were applied once as a ten-minute treatment to infested hair.[5] The benefit of abametapir in comparison to placebo was assessed after 1, 7 and 14 days by comparing the counts of participants in each group who were free of live lice.[5] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[7]
References
- 1 2 3 4 5 6 7 8 9 10 "DailyMed - XEGLYZE- abametapir lotion". dailymed.nlm.nih.gov. Archived from the original on 9 December 2021. Retrieved 3 November 2022.
- 1 2 3 4 5 6 7 "Xeglyze (abametapir) lotion, for topical use" (PDF). U.S. Food and Drug Administration (FDA). Dr. Reddy's Laboratories. Inc. Archived (PDF) from the original on 1 November 2022. Retrieved 25 July 2020.
- 1 2 "Abametapir". SPS - Specialist Pharmacy Service. 8 November 2016. Archived from the original on 19 October 2021. Retrieved 3 November 2022.
- 1 2 Sunder, Meera (July 2022). "Abametapir 0.74% (Xeglyze) for the Treatment of Head Lice". American Family Physician. pp. 91–92. Archived from the original on 23 July 2022. Retrieved 3 November 2022.
- 1 2 3 4 5 6 7 "Drug Trial Snapshot: Xeglyze". U.S. Food and Drug Administration (FDA). 24 July 2020. Archived from the original on 22 October 2020. Retrieved 6 August 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 Abametapir Professional Drug Facts. Accessed 6 May 2021.
- ↑ "New Drug Therapy Approvals 2020". U.S. Food and Drug Administration (FDA). 31 December 2020. Archived from the original on 18 January 2021. Retrieved 17 January 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
Further reading
- Bowles VM, VanLuvanee LJ, Alsop H, Hazan L, Shepherd K, Sidgiddi S, et al. (September 2018). "Clinical studies evaluating abametapir lotion, 0.74%, for the treatment of head louse infestation". Pediatr Dermatol. 35 (5): 616–621. doi:10.1111/pde.13612. PMC 6175393. PMID 29999197.
External links
| External sites: |
|
|---|---|
| Identifiers: |