Asbestos-related diseases
| Asbestos-related disease | |
|---|---|
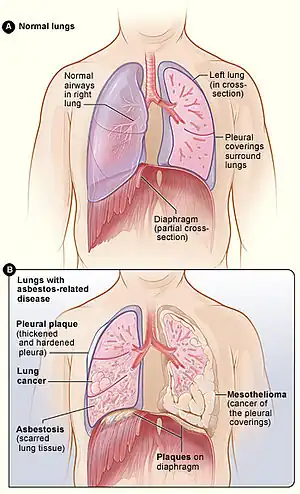 | |
| Figure A shows the location of the lungs, airways, pleura, and diaphragm in the body. Figure B shows lungs with asbestos-related diseases, including pleural plaque, lung cancer, asbestosis, plaque on the diaphragm, and mesothelioma. | |
| Specialty | Respirology |
Asbestos-related diseases are disorders of the lung and pleura caused by the inhalation of asbestos fibres. Asbestos-related diseases include non-malignant disorders such as asbestosis (pulmonary fibrosis due to asbestos), diffuse pleural thickening, pleural plaques, pleural effusion, rounded atelectasis and malignancies such as lung cancer and malignant mesothelioma.
People who worked in jobs with high asbestos dust exposure are at the highest risk of developing asbestos-related disease. However, exposure to asbestos may also occur in the worker’s home due to dust that has accumulated on the worker's clothing (para-occupational exposure). Asbestos-related diseases can also occur as a result of non-occupational, environmental exposure. Asbestos was extensively used in many building materials, therefore large quantities of asbestos still remain in buildings that were built prior to the restriction of asbestos use that applies in many countries. The weathering and aging of such buildings may cause asbestos fragments to be released in the air and create a potential hazard. Anyone who disturbs the asbestos-containing material during home maintenance and renovation can be affected,[1] although the exact risks are difficult to quantify.
Pathophysiology
Inhaled asbestos fibres enter the upper and lower respiratory tracts when asbestos is released into the air. Some of the inhaled fibers are cleared by the mucociliary clearance mechanism but long thin asbestos fibers may reach the lower airways and alveoli, and can be retained in the lungs for many years. Amphibole fibers are not cleared as effectively as serpentines and therefore accumulate more readily in the distal lung parenchyma.[2] Asbestos fibres are recognised by the lungs as foreign bodies and cause the activation of the lung’s local immune system leading to inflammation, cell and tissue damage. In the long term, this can lead to fibrosis, or rarely to malignancy. From the lungs, some asbestos fibres (mainly short fibres) can also migrate to pleural and peritoneal spaces.[3]
Non-malignant asbestos-related pleural diseases
Benign asbestos-related pleural abnormalities encompass four types of pleural changes:
- Pleural plaques
- Diffuse pleural thickening
- Benign asbestos pleural effusions
- Rounded atelectasis (folded lung)
The pleura appears to be more sensitive than the lung parenchyma to the effects of asbestos fibres.[4] Thus asbestos-related pleural diseases can result from much lower doses than the fibrotic changes in the lung.
Pleural plaques
Pleural plaques are the most common manifestation of asbestos exposure, affecting up to 58% of asbestos-exposed workers. The prevalence among the general population exposed environmentally ranges from 0.53 to 8%.[4] Pleural plaques are discrete circumscribed areas of hyaline fibrosis (patches of thickening) of the parietal pleura and rarely the visceral pleura that develop 20 to 40 years after first exposure. Over time, usually more than 30 years, they often become partly calcified. They consist of mature collagen fibers arranged in an open basket-weave pattern and are covered by flattened or cuboidal mesothelial cells.[5] They have a white or pale yellow shaggy appearance and are typically distributed on the posterolateral chest wall, diaphragm, and mediastinal pleura.[6] The number and size varies. Pleural plaques are typically asymptomatic, however, there is still some controversy on this topic. An association between pleural plaques and chest pain has been reported,[7] but this has not been confirmed in more recent studies.[8] Similarly, an association between pleural plaques and a restrictive impairment with diminished diffusing capacity on pulmonary function testing has been described.[9] This has not been a consistent finding and it has been postulated that this might be related to undetected early fibrosis.[5] The pathogenesis of pleural plaques remains uncertain. The most likely explanation is that asbestos fibres reach the parietal pleura by passage through lymphatic channels where they excite an inflammatory reaction.[4] The chest X-ray is the usual tool for diagnosing pleural plaques but chest CT scan is more sensitive and specific in this regard. Pleural plaques are evidence of past asbestos exposure and indicate an increased risk for the future development of other asbestos-related diseases. Pleural plaques in themselves are not pre-malignant. Individuals with pleural plaques are usually not compensated in most compensation systems.
Diffuse pleural thickening
Diffuse pleural thickening (DPT) is non-circumscribed fibrous thickening of the visceral pleura with areas of adherence to the parietal pleura and obliteration of the pleural space.[10] It often extends over the area of an entire lobe or lung, with fibrotic areas involving costophrenic angles, apices, lung bases, and interlobar fissures. The thickness ranges from less than 1 mm up to 1 cm or more and may extend for a few millimeters into the lung parenchyma.[5] Fibrous strands (“crow's feet”) extending from the thickened pleura into the lung parenchyma can be often detected on CT scan. Diffuse pleural thickening develops 20 to 40 years after first exposure.[11] All types of asbestos can cause diffuse pleural thickening and a dose-related relationship has been described.[6] It is thought that asbestos fibres that reach the pleura induce subpleural fibroblasts and mesothelial cells to produce scar tissue and collagen deposition, resulting in subpleural thickening.[6] Pleural plaques often coexist with DPT although the latter is rare compared with pleural plaques. According to the Australian Surveillance of Australian Workplace Based Respiratory Events (SABRE) scheme, DPT accounted for 22% of all asbestos-related diseases.[12] It usually begins with an inflammation of the pleura that is accompanied by a pleural effusion. Most patients complain of exertional breathlessness, however, chest pain has been also associated with this disorder.[10][11] DPT has a significant impact on pulmonary function, causing a decrease in forced vital capacity, reducing total lung capacity and diffusing capacity.[10][13] The restrictive impairment is a result of adhesions of the parietal with the visceral pleura as well as possible diaphragmatic involvement. Medical imaging is needed for diagnosis of diffuse pleural thickening. The appearance on a postero-anterior chest radiograph is of a continuous, irregular pleural shadowing. In accordance with the International Labour Organization (2000) classification, diffuse pleural thickening is considered to be present if there is obliteration of the costophrenic angle in continuity with ≥3 mm pleural thickening.[14] CT scanning is more sensitive than chest radiography and can detect early pleural thickening (i.e. 1-2mm in thickness).[6] The most commonly used classification system defines diffuse pleural thickening as a continuous sheet of pleural thickening more than 5 cm wide, more than 8 cm in craniocaudal extent, and more than 3 mm thick.[15] Most patients are only mildly impaired by diffuse pleural thickening. Treatment options are limited but any new onset or severe pain should be investigated to exclude malignancy. In most compensation systems, patients are eligible for compensation which corresponds to the severity of disability.
Benign asbestos pleural effusion
Benign asbestos pleural effusion is an exudative pleural effusion (a buildup of fluid between the two pleural layers) following asbestos exposure. It is relatively uncommon and the earliest manifestation of disease following asbestos exposure, usually occurring within 10 years from exposure. Effusions may be asymptomatic but rarely, they can cause pain, fever, and breathlessness.[5] Effusions usually last for 3–4 months and then resolve completely. They can also progress to diffuse pleural thickening. Diagnosis relies on a compatible history of asbestos exposure and exclusion of other probable causes.
Rounded atelectasis
Rounded atelectasis (also known as Blesovsky’s or folded lung syndrome) develops from infolding of thickened visceral pleura with collapse of the intervening lung parenchyma.[5] It presents radiographically as a mass and may be mistaken for a tumour. On a CT scan of the chest it appears as a rounded mass like opacity in the peripheral lung adjacent to thickened pleura and with curvilinear opacities which are the bronchi and vessels (comet tail).[16] Rounded atelectasis is the least common asbestos-related benign pleural disease. Exposure to asbestos is the most likely cause today but it can occur following other medical conditions. It is a chronic condition and usually asymptomatic.
Asbestosis
Asbestosis is a chronic lung disease caused by scarring of lung tissue, which results from prolonged exposure to asbestos. It is defined as diffuse interstitial pulmonary fibrosis secondary to asbestos exposure. It initially affects the lung bases and usually manifests after 15 or more years from initial exposure. It occurs after high intensity and/or long-term exposure to asbestos. Asbestos-related fibrosis is progressive because it continues to progress in the lung even if no further asbestos is inhaled. The scar tissue causes the alveolar walls to thicken, reducing the lung capacity which leads to the patient experiencing shortness of breath (dyspnea). Sufferers are at an increased risk for heart failure and certain malignancies.
Malignant asbestos-related diseases
Malignant mesothelioma
Malignant mesothelioma is an aggressive and incurable tumour caused by asbestos arising from mesothelial cells of the pleura, peritoneum (the lining of the abdominal cavity) and rarely elsewhere. Pleural mesothelioma is the most common type of mesothelioma, representing about 75 percent of cases. Peritoneal mesothelioma is the second most common type, consisting of about 10 to 20 percent of cases. Mesothelioma appears from 20 to 50 years after the initial exposure to asbestos. The symptoms include shortness of breath, chronic chest pain, cough, and weight loss. Diagnosing mesothelioma is often difficult and can include physical examination, chest X-ray and lung function tests, followed by CT scan and MRI. A biopsy is needed to confirm a diagnosis of malignant mesothelioma. Mesothelioma has a poor prognosis, with most patients dying within 1 year of diagnosis. The treatment strategies include surgery, radiotherapy, chemotherapy or multimodality treatment. Several tumour biomarkers (soluble mesothelin-related protein (SMRP),[17] osteopontin[18] and fibulin3[19]) have been evaluated for diagnostic purposes to allow early detection of this disease. Novel biomarkers such as volatile organic compounds measured in exhaled breath are also promising.[20]
Asbestos-related lung cancer
Asbestos can cause lung cancer that is identical to lung cancer from other causes. Exposure to asbestos is associated with all major histological types of lung carcinoma (adenocarcinoma, squamous cell carcinoma, large-cell carcinoma and small-cell carcinoma). The latency period between exposure and development of lung cancer is 20 to 30 years. It is estimated that 3–8% of all lung cancers are related to asbestos.[21] The risk of developing lung cancer depends on the level, duration, and frequency of asbestos exposure (cumulative exposure). Smoking and individual susceptibility are other contributing factors towards lung cancer. Smokers who have been exposed to asbestos are at far greater risk of lung cancer. Smoking and asbestos exposure have a multiplicative (synergistic) effect on the risk of lung cancer. Symptoms include chronic cough, chest pain, breathlessness, haemoptysis (coughing up blood), wheezing or hoarseness of the voice, weight loss and fatigue. Treatment involves surgical removal of the cancer, chemotherapy, radiotherapy, or a combination of these (multimodality treatment). Prognosis is generally poor unless the cancer is detected in its early stages. Out of all patients diagnosed with lung cancer, only 15% survive for five years after diagnosis.
History
Thousands of scientific and medical articles have chronicled human understanding of the hazards of asbestos to human life.[22] This understanding paralleled the growth of the industrial revolution, particularly in the textile factories and mines of Great Britain. This body of knowledge is frequently referred to in litigation as the state of the art or the benchmark for determining if a company acted within the bounds of negligent behavior. The following is a chronological list of some of the major pre-1950 scientific and medical articles relating to the knowledge of the medical and scientific communities regarding asbestos and disease in humans:
| Year | Publication |
|---|---|
| 1898 | "Annual Report of the Chief Inspector of Factories and Workshops, Part II". H.M. Stationery Office. 1898: 171–172. {{cite journal}}: Cite journal requires |journal= (help) |
| 1912 | "Effect of Asbestos Dust on Workers Health in Asbestos Mines and Factories". The Labour Gazette: 761–762. 1912. |
| 1918 | Hoffman, F.L. (1918). Mortality from Respiratory Diseases in Dusty Trades (Inorganic Dusts). U.S. Dept. of Labor, Bureau of Labor Statistics. pp. 35–47, 163–181. |
| 1924 | Cooke, W.E. (July 26, 1924). "Fibrosis of the Lungs due to the Inhalation of Asbestos Dust". British Medical Journal. 2 (3317): 147–140.2. doi:10.1136/bmj.2.3317.147. PMC 2304688. PMID 20771679. |
| 1928 | Editorial (1928). "Pulmonary Asbestosis". JAMA. 90 (2): 119–120. doi:10.1001/jama.1928.02690290049014. |
| 1928 | Simpson, F.W. (1929). "Pulmonary Asbestosis in South Africa". British Medical Journal. 1 (3516): 885–887. doi:10.1136/bmj.1.3516.885. PMC 2455583. |
| 1929 | Haddow, A.C. (August 3, 1929). "Asbestosis". The Lancet. 214: 231. doi:10.1016/s0140-6736(01)04102-2. |
| 1929 | Wood, W. Burton (May 1929). "Pulmonary asbestosis: Radiographic appearances in skiagrams of the chests of workers in asbestos". Tubercle. 10 (8): 353–363. doi:10.1016/S0041-3879(29)80024-4. |
| 1930 | Correspondence, Foreign Letters (June 28, 1930). "Compensation Act to be Extended to Asbestosis". JAMA. 94 (26): 2078. doi:10.1001/jama.1930.02710520044016. |
| 1930 | Mills, R.G. (June 28, 1930). "Report of a Case". Minnesota Medicine: 495–499. |
| 1930 | Editorial (1930). "Current Comment, Pulmonary Asbestosis". JAMA. 95 (19): 1431. doi:10.1001/jama.1930.02720190042014. |
| 1930 | Merewether, E.R.A. (May 1930). "The Occurrence of Pulmonary Fibrosis and Other Pulmonary Afflictions in Asbestos Workers". J.Indus.Hyg. 5. 12: 198–257. |
| 1930 | "Health and Industrial Hygiene - Pulmonary Asbestosis". Monthly Labor Review. 31: 74–76. 1930. |
| 1930 | Encyclopedia of Hygiene, Pathology and Social Welfare: Occupation and Health, Vol. I, A-H. Geneva: International Labor Office. 1930. pp. 189–181. |
| 1930 | Gardner, L.U. (1931). "Studies on Experimental Pneumonoconiosis: VI. Inhalation of Asbestos Dust, Its Effect Upon Primary Tuberculosis Infection". J.Indus.Hyg. 2. 13: 65–114. |
| 1930 | Gordon, B (June 1931). "Pulmonary Asbestosis". Penn.Med.J. 35: 637–639. |
| 1934 | Woods, W.B.; Gloyne, S.R. (1934). "Pulmonary Asbestosis". Lancet. 2 (5808): 1383–1385. doi:10.1016/s0140-6736(00)43332-5. |
| 1938 | Dreesen (August 1938). "A Study of Asbestos in the Asbestos Textile Industry". U.S. Treasury Dept., Public Health Bulletin: 1–126. |
| 1941 | Dublin (1941). "Occupational Hazards and Diagnostic Signs, Bulletin". U.S. Dept. Of Labor, Div. Of Labor Standards. 41: II, IV, V and 25. |
| 1942 | Holleb, H.B. (1942). "Bronchiogenic Carcinoma in Association with Pulmonary Asbestosis". American Journal of Pathology: 123–131. |
| 1944 | Wedler, H.W. (1944). "Asbestosis and Pulmonmary Carcinoma". Bulletin of Hygiene. 19: 362. |
| 1944 | Editorial (November 25, 1944). "Environmental Cancer". JAMA. 126 (13): 836. doi:10.1001/jama.1944.02850480036012. |
| 1944 | Hutchinson (1944). "Dust as an Industrial Health Hazard". Heating and Ventilating. 41 (6): 57–61. |
| 1946 | Fleischer, W.F. (1946). "Health Survey of Pipe Covering Operations in Constructing Naval Vessels". Journal of Industrial Hygiene and Toxicology. 1: 9–16. PMID 21016030. |
| 1948 | Lynch, K.M. (1948). "Asbestosis IV: Analysis of Forty Necropsied Cases, Diseases of the Chest": 79–81. {{cite journal}}: Cite journal requires |journal= (help) |
| 1949 | Merewether (1949). "Annual Report of the Chief Inspector of Factories for 1947". London: H.M. Stationary Ofc.: 79–81. {{cite journal}}: Cite journal requires |journal= (help) |
| 1949 | Wyers (1949). "Asbestosis". Postgraduate Medical Journal. 25 (290): 631–638. doi:10.1136/pgmj.25.290.631. PMC 2530167. PMID 15396262. |
References
- ↑ Olsen NJ, Franklin PJ, Reid A, et al. (2011). "Increasing incidence of malignant mesothelioma after exposure to asbestos during home maintenance and renovation". Medical Journal of Australia. 195 (5): 271–274. doi:10.5694/mja11.10125. PMID 21895596.
- ↑ Kamp D.W. (2009). "Asbestos-induced lung diseases: an update". Translational Research. 153 (4): 143–52. doi:10.1016/j.trsl.2009.01.004. PMC 3544481. PMID 19304273.
- ↑ Broaddus VC (May 2001). "Apoptosis and asbestos-induced disease: Is there a connection?". The Journal of Laboratory and Clinical Medicine. 137 (5): 314–5. doi:10.1067/mlc.2001.115172. PMID 11329527.
- 1 2 3 Peacock, C., S.J. Copley, and D.M. Hansell, Asbestos-related benign pleural disease. Clinical Radiology, 2000. 55(6): p. 422-32.
- 1 2 3 4 5 American Thoracic Society. Diagnosis and Initial Management of Nonmalignant Diseases Related to Asbestos" American Journal of Respiratory and Critical Care Medicine 2004;170:691-715
- 1 2 3 4 Miles SE, Sandrini A, Johnson AR, Yates DH Clinical consequences of asbestos-related diffuse pleural thickening: A review" Journal of Occupational Medicine and Toxicology 2008;3:20
- ↑ Mukherjee S, de Klerk N, Palmer LJ, et al. (2000). "Chest pain in asbestos-exposed individuals with benign pleural and parenchymal disease". American Journal of Respiratory and Critical Care Medicine. 162 (5): 1807–1811. doi:10.1164/ajrccm.162.5.9912012. PMID 11069817.
- ↑ Park EK, Thomas PS, Wilson D, et al. (2011). "Chest pain in asbestos and silica-exposed workers". Occupational Medicine. 61 (3): 178–183. doi:10.1093/occmed/kqr011. PMID 21406408.
- ↑ Oliver LC, Eisen EA, Greene R, Sprince NL. Asbestos-related pleural plaques and lung function" American Journal of Industrial Medicine 1988;14:649–656.
- 1 2 3 Yates D.H.; et al. (1996). ""Asbestos-related bilateral diffuse pleural thickening " natural history of radiographic and lung function abnormalities". American Journal of Respiratory and Critical Care Medicine. 153 (1): 301–6. doi:10.1164/ajrccm.153.1.8542134. PMID 8542134.
- 1 2 Jeebun V, Stenton SC (2012). "The presentation and natural history of asbestos-induced diffuse pleural thickening". Occupational Medicine. 62 (4): 266–268. doi:10.1093/occmed/kqs028. PMID 22539640.
- ↑ Hannaford-Turner K, Elder D, Sim MR, Abramson MJ, Johnson AR, Yates DH (Aug 2010). "Surveillance of Australian workplace Based Respiratory Events (SABRE) in New South Wales". Occupational Medicine. 60 (5): 376–82. doi:10.1093/occmed/kqq011. PMID 20308261.
- ↑ Kee ST, Gamsu G, Blanc P. Causes of pulmonary impairment in asbestos- exposed individuals with diffuse pleural thickening" American Journal of Respiratory and Critical Care Medicine 1996;154:789–793
- ↑ International Labor Office International, Classification of Radiographs of Pneumoconioses. Geneva, Switzerland: International Labour Organization; 2011.
- ↑ Lynch, DA; Gamsu, G; Aberle, DR (1989). "Conventional and high resolution computed tomography in the diagnosis of asbestos-related diseases". Radiographics. 9 (3): 523–51. doi:10.1148/radiographics.9.3.2727359. PMID 2727359.
- ↑ Batra, P., et al., Rounded atelectasis. Journal of Thoracic Imaging, 1996. 11(3): p. 187-97.
- ↑ Park EK, Sandrini A, Yates DH, et al. (2008). "Soluble mesothelin-related protein in an asbestos-exposed population: the dust diseases board cohort study". American Journal of Respiratory and Critical Care Medicine. 178 (8): 832–837. doi:10.1164/rccm.200802-258oc. PMID 18583574.
- ↑ Park EK, Thomas PS, Johnson AR, Yates DH (2009). "Osteopontin levels in an asbestos-exposed population". Clinical Cancer Research. 15 (4): 1362–1366. doi:10.1158/1078-0432.ccr-08-0360. PMID 19174489.
- ↑ Pass HI, Levin SM, Harbut MR, et al. (2012). "Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma". The New England Journal of Medicine. 367 (15): 1417–1427. doi:10.1056/nejmoa1115050. PMC 3761217. PMID 23050525.
- ↑ Chapman EA, Thomas PS, Stone E, et al. (2012). "A breath test for malignant mesothelioma using an electronic nose". The European Respiratory Journal. 40 (2): 448–54. doi:10.1183/09031936.00040911. PMID 22183490.
- ↑ McCormack V.; et al. (2012). "Estimating the asbestos-related lung cancer burden from mesothelioma mortality". British Journal of Cancer. 106 (3): 575–84. doi:10.1038/bjc.2011.563. PMC 3273352. PMID 22233924.
- ↑ Lemen, Richard; Dement (Feb 1980). "Epidemiology of asbestos-related diseases". Environ. Health Perspect. 34: 1–11. doi:10.1289/ehp.80341. PMC 1568524. PMID 6993197.