Atypical ductal hyperplasia
| Atypical ductal hyperplasia | |
|---|---|
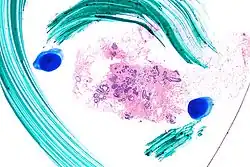 | |
| Very low magnification micrograph of atypical ductal hyperplasia (ADH). The piece with ADH was circled by the pathologist with a marker, as it is so small, and sent for an additional opinion. H&E stain. | |
| Specialty | Gynecology, pathology |
Atypical ductal hyperplasia (ADH) is the term used for a benign lesion of the breast that indicates an increased risk of breast cancer.[1]
The name of the entity is descriptive of the lesion; ADH is characterized by cellular proliferation (hyperplasia) within one or two breast ducts and (histomorphologic) architectural abnormalities, i.e. the cells are arranged in an abnormal or atypical way.
In the context of a core (needle) biopsy, ADH is considered an indication for a breast lumpectomy, also known as a surgical (excisional) biopsy, to exclude the presence of breast cancer.[2]
Signs and symptoms
ADH, generally, is asymptomatic. It usually comes to medical attention on a screening mammogram, as a non-specific suspicious abnormality that requires a biopsy.
Pathology
ADH, cytologically, architecturally and on a molecular basis, is identical to a low-grade ductal carcinoma in situ (DCIS);[3] however, it has a limited extent, i.e. is present in a very small amount (< 2 mm).
 Low mag.
Low mag. High mag.
High mag.
Relation to low-grade ductal carcinoma in situ
While the histopathologic features and molecular features of ADH are that of (low-grade) DCIS, its clinical behaviour, unlike low-grade DCIS, is substantially better; thus, the more aggressive treatment for DCIS is not justified.
Diagnosis

- A - One focus (< 2 mm) of two architecturally disarranged cross sections of tubuli showing a monotonous intraductal proliferation with secondary intraluminal architecture. Hematoxylin and Eosin stain.
- B - One area of an ADH with associated calcifications intraluminal. Hematoxylin and Eosin stain.
- C - Higher magnification of ADH shows low-grade nuclear atypia and monotonous cell proliferation along with secondary intraluminal architecture. Hematoxylin and Eosin stain.
- D - Strong and uniform expression of estrogen receptors (ER). ER immunohistochemistry.
- E - Lack of basal cytokeratins (CK5/6). CK5/6 immunohistochemistry.
It is diagnosed based on tissue, e.g. a biopsy,[5] showing ductal hyperplasia.
There is no single definite cutoff that separates atypical ductal hyperplasia from ductal carcinoma in situ, but the following are important distinctive features of atypical ductal hyperplasia, with suggested cutoffs:[6]
- Size less than 2 mm.
- Not involving more than one duct.
- The atypical epithelial proliferation is admixed with a second population of proliferative cells without atypia.
- The proliferation completely involves the terminal ductal lobular unit(s), to a limited extent.
Treatment
ADH, if found on a surgical (excisional) biopsy of a mammographic abnormality, does not require any further treatment, only mammographic follow-up.
If ADH is found on a core (needle) biopsy (a procedure which generally does not excise a suspicious mammographic abnormality), a surgical biopsy, i.e. a breast lumpectomy, to completely excise the abnormality and exclude breast cancer is the typical recommendation.
Prognosis
Cancer risk for ADH on a core biopsy
The rate at which breast cancer (ductal carcinoma in situ or invasive mammary carcinoma (all breast cancer except DCIS and LCIS)) is found at the time of a surgical (excisional) biopsy, following the diagnosis of ADH on a core (needle) biopsy varies considerably from hospital-to-hospital (range 4-54%).[7] In two large studies, the conversion of an ADH on core biopsy to breast cancer on surgical excision, known as "up-grading", is approximately 30%.[7][8]
Cancer risk based on follow-up
The relative risk of breast cancer based on a median follow-up of 8 years, in a case control study of US registered nurses, is 3.7.[9]
See also
References
- ↑ "Understanding Breast Changes - National Cancer Institute". Archived from the original on May 27, 2010.
- ↑ Liberman L, Cohen MA, Dershaw DD, Abramson AF, Hann LE, Rosen PP (May 1995). "Atypical ductal hyperplasia diagnosed at stereotaxic core biopsy of breast lesions: an indication for surgical biopsy". AJR Am J Roentgenol. 164 (5): 1111–3. doi:10.2214/ajr.164.5.7717215. PMID 7717215.
- ↑ Ghofrani, M.; Tapia, B.; Tavassoli, FA. (Dec 2006). "Discrepancies in the diagnosis of intraductal proliferative lesions of the breast and its management implications: results of a multinational survey". Virchows Arch. 449 (6): 609–16. doi:10.1007/s00428-006-0245-y. PMC 1888715. PMID 17058097.
- ↑ Rageth, Christoph J.; Rubenov, Ravit; Bronz, Cristian; Dietrich, Daniel; Tausch, Christoph; Rodewald, Ann-Katrin; Varga, Zsuzsanna (2018). "Atypical ductal hyperplasia and the risk of underestimation: tissue sampling method, multifocality, and associated calcification significantly influence the diagnostic upgrade rate based on subsequent surgical specimens". Breast Cancer. 26 (4): 452–458. doi:10.1007/s12282-018-00943-2. ISSN 1340-6868. PMC 6570781. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)
- ↑ Eby PR, Ochsner JE, DeMartini WB, Allison KH, Peacock S, Lehman CD (January 2009). "Frequency and upgrade rates of atypical ductal hyperplasia diagnosed at stereotactic vacuum-assisted breast biopsy: 9-versus 11-gauge". AJR Am J Roentgenol. 192 (1): 229–34. doi:10.2214/AJR.08.1342. PMID 19098204.
- ↑ Tozbikian, Gary; Brogi, Edi; Vallejo, Christina E.; Giri, Dilip; Murray, Melissa; Catalano, Jeffrey; Olcese, Cristina; Van Zee, Kimberly J.; Wen, Hannah Yong (2016). "Atypical Ductal Hyperplasia Bordering on Ductal Carcinoma In Situ". International Journal of Surgical Pathology. 25 (2): 100–107. doi:10.1177/1066896916662154. ISSN 1066-8969. PMC 5285492.
- 1 2 Deshaies, I.; Provencher, L.; Jacob, S.; Côté, G.; Robert, J.; Desbiens, C.; Poirier, B.; Hogue, JC.; Vachon, E. (Feb 2011). "Factors associated with upgrading to malignancy at surgery of atypical ductal hyperplasia diagnosed on core biopsy". Breast. 20 (1): 50–5. doi:10.1016/j.breast.2010.06.004. PMID 20619647.
- ↑ Margenthaler, JA.; Duke, D.; Monsees, BS.; Barton, PT.; Clark, C.; Dietz, JR. (Oct 2006). "Correlation between core biopsy and excisional biopsy in breast high-risk lesions". Am J Surg. 192 (4): 534–7. doi:10.1016/j.amjsurg.2006.06.003. PMID 16978969.
- ↑ London, SJ.; Connolly, JL.; Schnitt, SJ.; Colditz, GA. (Feb 1992). "A prospective study of benign breast disease and the risk of breast cancer". JAMA. 267 (7): 941–4. doi:10.1001/jama.1992.03480070057030. PMID 1734106.