Tiomolibdic acid
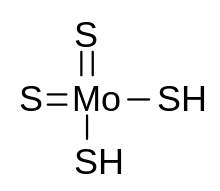 | |
| Clinical data | |
|---|---|
| Trade names | Decuprate |
| Other names | Tetrathiomolybdic acid; choline salt: ATN-224, WTX101 |
| ATC code |
|
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | H2MoS4 |
| Molar mass | 226.21 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
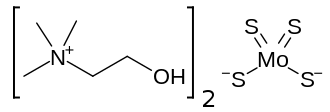
Tiomolibdic acid (trade name Decuprate) is a chelating agent under investigation for the treatment of cancer and of Wilson's disease,[1] a rare and potentially fatal disease in which the body cannot regulate copper. It is developed by Wilson Therapeutics and used in form of the salt bis-choline tetrathiomolybdate.
Wilson's disease is an autosomal recessive genetic disorder that is manifested by serious hepatic, neurologic or psychiatric symptoms. The disease is fatal if left untreated. It is estimated that approximately 1 individual in every 30,000 worldwide has Wilson's disease, corresponding to approximately 15,000 individuals in the European Union and approximately 11,000 in the United States.[2]
Bis-choline tetrathiomolybdate has been evaluated in clinical trials in patients with various forms of cancer[3][4][5] and has received orphan designation in the US and EU as a potential therapy against Wilson's disease.[6][7]
Pharmacology
Mechanism of action
Tiomolibdic acid selectively forms highly stable complexes with copper and proteins. These complexes are then believed to be primarily excreted via the bile, restoring the normal excretion route of copper that is impaired in patients with Wilson's disease.[8][9][10]
The binding and excretion mechanism is stable; whereas many de-coppering agents form unstable complexes that are excreted via urine.[11]
Clinical trials
As of November 2014, a Phase 2, multi-centre, open-label study was recruiting newly diagnosed Wilson's disease patients 18 and older to evaluate the efficacy and safety of bis-choline tetrathiomolybdate administration over a 24-week period.[12][13]
As of 2016, tetrathiomolybdate had been tested in over 500 patients for up to seven years, primarily in oncology[3][4][5][14][15][16][17][18][19][20] and Wilson's disease,[21][22][23][24] as well as some other clinical pathologies.[25][26]
The data suggest that bis-choline tetrathiomolybdate can rapidly lower and control toxic free copper levels and improve clinical symptoms in Wilson's disease patients. The data also suggest that it is generally well tolerated, with the potential for a reduced risk of neurological worsening after initiation of therapy compared to existing therapies.[22][23][24]
Dosing
Previous clinical studies with bis-choline tetrathiomolybdate in oncology patients have shown that it can lower and maintain copper levels using a once or twice daily oral dosing.[4][5] This may be helpful since untreated Wilson's disease may lead to death within several years of the onset of symptoms,[27] and medication use should continue throughout the patient's lifespan. Patient compliance is crucial for clinical improvement, and it is a particular challenge for Wilson's disease patients taking de-coppering treatments.[28]
Society and culture
Names
Tiomolibdic acid is the recommended International nonproprietary name (INN).[29]
References
- ↑ "Tiomolibdic acid". National Center for Advancing Translational Sciences. Retrieved 30 December 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML (February 2007). "Wilson's disease". Lancet. 369 (9559): 397–408. doi:10.1016/S0140-6736(07)60196-2. PMID 17276780. S2CID 24663871.
- 1 2 Berenson JR, Boccia RV, Bashey A, Levine AM, Koc ON, Callahan JA, Mazar AP, Reich SD (2006). "Phase I Study of the [Cu, Zn] Superoxide Dismutase (SOD1) Inhibitor ATN-224 (Bis-Choline Tetrathiomolybdate) in Patients with Advanced Hematologic Malignancies Presentation at the Amer Soc Hematol 2006 Annual Meeting". Blood. 108. Abstract 2593.
- 1 2 3 Lin J, Zahurak M, Beer TM, Ryan CJ, Wilding G, Mathew P, et al. (July 2013). "A non-comparative randomized phase II study of 2 doses of ATN-224, a copper/zinc superoxide dismutase inhibitor, in patients with biochemically recurrent hormone-naïve prostate cancer". Urologic Oncology. 31 (5): 581–8. doi:10.1016/j.urolonc.2011.04.009. PMC 3227793. PMID 21816640.
- 1 2 3 Lowndes SA, Adams A, Timms A, Fisher N, Smythe J, Watt SM, et al. (November 2008). "Phase I study of copper-binding agent ATN-224 in patients with advanced solid tumors". Clinical Cancer Research. 14 (22): 7526–34. doi:10.1158/1078-0432.CCR-08-0315. PMID 19010871.
- ↑ Public summary of opinion on orphan designation: Choline tetrathiomolybdate for the treatment of Wilson's disease, EMA/COMP/795268/2012, ATN-224, 18 February 2013.
- ↑ Orphan Drug Designations and Approvals: choline tetrathiomolybdate, U.S. Food and Drug Administration, 25 August 2011
- ↑ Komatsu Y, Sadakata I, Ogra Y, Suzuki KT (February 2000). "Excretion of copper complexed with thiomolybdate into the bile and blood in LEC rats". Chemico-Biological Interactions. 124 (3): 217–31. doi:10.1016/s0009-2797(99)00159-3. PMID 10728780.
- ↑ McQuaid A, Mason J (February 1991). "A comparison of the effects of penicillamine, trientine, and trithiomolybdate on [35S]-labeled metallothionein in vitro; implications for Wilson's disease therapy". Journal of Inorganic Biochemistry. 41 (2): 87–92. doi:10.1016/0162-0134(91)80002-y. PMID 2033396.
- ↑ Ogra Y, Ohmichi M, Suzuki KT (October 1995). "Systemic dispositions of molybdenum and copper after tetrathiomolybdate injection in LEC rats". Journal of Trace Elements in Medicine and Biology. 9 (3): 165–9. doi:10.1016/S0946-672X(11)80042-8. PMID 8605606.
- ↑ Říha M, Karlíčková J, Filipský T, Macáková K, Hrdina R, Mladěnka P (June 2013). "Novel method for rapid copper chelation assessment confirmed low affinity of D-penicillamine for copper in comparison with trientine and 8-hydroxyquinolines". Journal of Inorganic Biochemistry. 123: 80–7. doi:10.1016/j.jinorgbio.2013.02.011. PMID 23563391.
- ↑ Clinical trial number NCT02273596 for "Efficacy and Safety Study of WTX101 in Adult Wilson Disease Patients" at ClinicalTrials.gov
- ↑ "Wilson Disease Clinical Trials. Phase 2 Study in Newly Diagnosed Wilson Disease Patients with WTX101 (Tetrathiomolybdate)". Wilson Disease Association. 2009. Archived from the original on 9 February 2015.
- ↑ Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, et al. (January 2000). "Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study". Clinical Cancer Research. 6 (1): 1–10. PMID 10656425.
- ↑ Gartner EM, Griffith KA, Pan Q, Brewer GJ, Henja GF, Merajver SD, Zalupski MM (April 2009). "A pilot trial of the anti-angiogenic copper lowering agent tetrathiomolybdate in combination with irinotecan, 5-flurouracil, and leucovorin for metastatic colorectal cancer". Investigational New Drugs. 27 (2): 159–65. doi:10.1007/s10637-008-9165-9. PMC 4171042. PMID 18712502.
- ↑ Henry NL, Dunn R, Merjaver S, Pan Q, Pienta KJ, Brewer G, Smith DC (2006). "Phase II trial of copper depletion with tetrathiomolybdate as an antiangiogenesis strategy in patients with hormone-refractory prostate cancer". Oncology. 71 (3–4): 168–75. doi:10.1159/000106066. PMID 17641535. S2CID 25861052.
- ↑ Jain S, Cohen J, Ward MM, Kornhauser N, Chuang E, Cigler T, et al. (June 2013). "Tetrathiomolybdate-associated copper depletion decreases circulating endothelial progenitor cells in women with breast cancer at high risk of relapse". Annals of Oncology. 24 (6): 1491–8. doi:10.1093/annonc/mds654. PMC 3707432. PMID 23406736.
- ↑ Pass HI, Brewer GJ, Dick R, Carbone M, Merajver S (August 2008). "A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: final results". The Annals of Thoracic Surgery. 86 (2): 383–9, discussion 390. doi:10.1016/j.athoracsur.2008.03.016. PMID 18640301.
- ↑ Redman BG, Esper P, Pan Q, Dunn RL, Hussain HK, Chenevert T, et al. (May 2003). "Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer". Clinical Cancer Research. 9 (5): 1666–72. PMID 12738719.
- ↑ Schneider BJ, Lee JS, Hayman JA, Chang AC, Orringer MB, Pickens A, et al. (April 2013). "Pre-operative chemoradiation followed by post-operative adjuvant therapy with tetrathiomolybdate, a novel copper chelator, for patients with resectable esophageal cancer". Investigational New Drugs. 31 (2): 435–42. doi:10.1007/s10637-012-9864-0. PMC 4418641. PMID 22847786.
- ↑ Roberts EA, Schilsky ML (June 2008). "Diagnosis and treatment of Wilson disease: an update". Hepatology. 47 (6): 2089–111. doi:10.1002/hep.22261. PMID 18506894.
- 1 2 Brewer GJ, Askari F, Dick RB, Sitterly J, Fink JK, Carlson M, et al. (August 2009). "Treatment of Wilson's disease with tetrathiomolybdate: V. Control of free copper by tetrathiomolybdate and a comparison with trientine". Translational Research. 154 (2): 70–7. doi:10.1016/j.trsl.2009.05.002. PMID 19595438.
- 1 2 Brewer GJ, Askari F, Lorincz MT, Carlson M, Schilsky M, Kluin KJ, et al. (April 2006). "Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease". Archives of Neurology. 63 (4): 521–7. doi:10.1001/archneur.63.4.521. PMID 16606763.
- 1 2 Brewer GJ, Hedera P, Kluin KJ, Carlson M, Askari F, Dick RB, et al. (March 2003). "Treatment of Wilson disease with ammonium tetrathiomolybdate: III. Initial therapy in a total of 55 neurologically affected patients and follow-up with zinc therapy". Archives of Neurology. 60 (3): 379–85. doi:10.1001/archneur.60.3.379. PMID 12633149.
- ↑ Askari F, Innis D, Dick RB, Hou G, Marrero J, Greenson J, Brewer GJ (March 2010). "Treatment of primary biliary cirrhosis with tetrathiomolybdate: results of a double-blind trial". Translational Research. 155 (3): 123–30. doi:10.1016/j.trsl.2009.09.009. PMID 20171597.
- ↑ Vine AK, Brewer GJ (2002). "Tetrathiomolybdate as an antiangiogenesis therapy for subfoveal choroidal neovascularization secondary to age-related macular degeneration". Transactions of the American Ophthalmological Society. 100: 73–6, discussion 76-7. PMC 1358949. PMID 12545680.
- ↑ Członkowska A, Tarnacka B, Litwin T, Gajda J, Rodo M (June 2005). "Wilson's disease-cause of mortality in 164 patients during 1992-2003 observation period". Journal of Neurology. 252 (6): 698–703. doi:10.1007/s00415-005-0720-4. PMID 15742108. S2CID 34212689.
- ↑ Masełbas W, Chabik G, Członkowska A (2010). "Persistence with treatment in patients with Wilson disease". Neurologia I Neurochirurgia Polska. 44 (3): 260–3. doi:10.1016/s0028-3843(14)60040-2. PMID 20625962.
- ↑ World Health Organization (2010). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 63". WHO Drug Information. 24 (1). hdl:10665/74530.
External links
- "Tiomolibdic acid". Drug Information Portal. U.S. National Library of Medicine.