Pegvaliase
 | |
| Names | |
|---|---|
| Pronunciation | peg val' i ase |
| Trade names | Palynziq |
| Other names | Pegvaliase-pqpz; PEG-PAL; RAvPAL-PEG |
IUPAC name
| |
| Clinical data | |
| Drug class | Enzyme[1] |
| Main uses | Phenylketonuria[1] |
| Side effects | Pain at injection site, joint pain, allergic reactions[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Subcutaneous |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
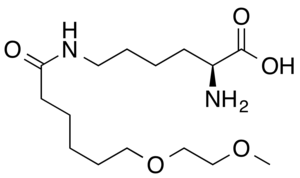
| Formula | C15H30N2O5 |
| Molar mass | 318.414 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pegvaliase, sold under the brand name Palynziq, is a medication used to treat phenylketonuria.[1] It is used when other treatments, including diet and sapropterin, are not sufficient.[1][3] It is given by injection under the skin.[3]
Common side effects include pain at the site of injection, joint pain, and allergic reactions.[1] Other side effects may include anaphylaxis, angioedema, and serum sickness.[1][3] It is unclear if use during pregnancy is safe for the baby.[3] It is the enzyme phenylalanine ammonia lyase connected to polyethylene glycol and works by breaking down phenylalanine.[1][3]
Pegvaliase was approved for medical use in the United States in 2018 and Europe in 2019.[3][1] It is not available in the United Kingdom as of 2021.[4] In the United States it costs about 560 USD per dose up to 20 mg as of 2021.[5]
Medical uses
Dosage
It is generally started at a dose of 2.5 mg once a week for 4 weeks.[1]
This may be increased up to 60 mg once per day until phenylalanine blood levels are controlled.[1]
History
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[6]
References
- 1 2 3 4 5 6 7 8 9 10 11 "Palynziq". Archived from the original on 20 October 2021. Retrieved 27 October 2021.
- 1 2 "Palynziq". Therapeutic Goods Administration (TGA). 23 July 2021. Archived from the original on 5 September 2021. Retrieved 5 September 2021.
- 1 2 3 4 5 6 "Pegvaliase-pqpz Monograph for Professionals". Drugs.com. Retrieved 27 October 2021.
- ↑ "Pegvaliase". SPS - Specialist Pharmacy Service. 31 March 2015. Archived from the original on 27 October 2021. Retrieved 27 October 2021.
- ↑ "Palynziq Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 26 January 2021. Retrieved 27 October 2021.
- ↑ New Drug Therapy Approvals 2018 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2019. Archived from the original on 17 September 2020. Retrieved 16 September 2020.
External links
| External sites: |
|
|---|---|
| Identifiers: |