Bacterial capsule
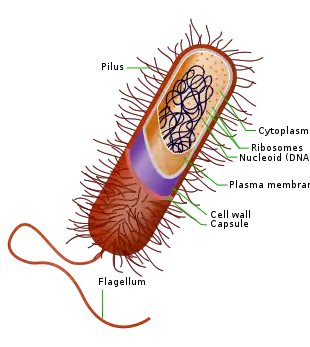
The bacteria capsule is a large structure common to many bacteria.[1] It is a polysaccharide layer that lies outside the cell envelope, and is thus deemed part of the outer envelope of a bacterial cell. It is a well-organized layer, not easily washed off, and it can be the cause of various diseases.[2][3]
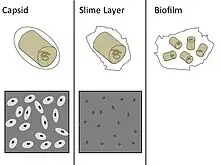
The capsule—which can be found in both gram negative and gram-positive bacteria—is different from the second lipid membrane – bacterial outer membrane, which contains lipopolysaccharides and lipoproteins and is found only in gram-negative bacteria. When the amorphous viscid secretion (that makes up the capsule) diffuses into the surrounding medium and remains as a loose undemarcated secretion, it is known as a slime layer. Capsule and slime layer are sometimes summarized under the term glycocalyx.
Composition
Most bacterial capsules are composed of polysaccharide,[4] but some species use other materials, such as poly-D-glutamic acid in Bacillus anthracis. Because most capsules are so tightly packed, they are difficult to stain because most standard stains cannot penetrate the capsule. To visualize encapsulated bacteria using a microscope, a sample is treated with a dark stain, such as India ink. The structure of the capsule prevents the stain from penetrating the cell. When viewed, bacterial capsules appear as a bright halo around the cell on a dark background.[5]
Function
The capsule is considered a virulence factor because it enhances the ability of bacteria to cause disease (e.g. prevents phagocytosis). The capsule can protect cells from engulfment by eukaryotic cells, such as macrophages.[6] A capsule-specific antibody may be required for phagocytosis to occur. Capsules also contain water which protects the bacteria against desiccation. They also exclude bacterial viruses and most hydrophobic toxic materials such as detergents. Immunity to one capsule type does not result in immunity to the other types. Capsules also help cells adhere to surfaces. As a group where the capsule is present they are known as polysaccharide encapsulated bacteria or encapsulated bacteria.[7]
Diversity
The capsule is found most commonly among gram-negative bacteria:
- Escherichia coli (in some strains)
- Neisseria meningitidis[8][9][10]
- Klebsiella pneumoniae[11][12][13]
- Haemophilus influenzae[14]
- Pseudomonas aeruginosa[15]
- Salmonella[16]
- Acinetobacter baumannii[17][18]
However, some gram-positive bacteria may also have a capsule:
- Bacillus megaterium for example, synthesizes a capsule composed of polypeptide and polysaccharides.
- Bacillus anthracis
- Streptococcus pyogenes synthesizes a hyaluronic acid capsule.
- Streptococcus pneumoniae[19] has at least 91 different capsular serotypes.[20] These serotypes are the basis for the pneumococcal vaccines.
- Streptococcus agalactiae produces a polysaccharide capsule of nine antigenic types that all contain sialic acid (Ia, Ib, II, III, IV, V, VI, VII, VIII).
- Staphylococcus epidermidis
- Staphylococcus aureus
The yeast Cryptococcus neoformans,[21] though not a bacterium, has a similar capsule.[22][23]
Capsules too small to be seen with an ordinary microscope, such as the M protein of Streptococcus pyogenes, are called microcapsules.
Demonstration of capsule
- India ink staining: the capsule appears as a clear halo around the bacterium as the ink can't penetrate the capsule.[24]: 87
- Maneval's capsule stain: the capsule appears as a clear halo between the pink-stained bacterium and the bluish-grey stained background. The background stain is the acidic stain Congo red (which changes color to bluish-grey due to the pH), and the pink stain is acid fuschin.
- Serological methods: Capsular material is antigenic and can be demonstrated by mixing it with a specific anticapsular serum. When examined under the microscope, the capsule appears 'swollen' due to an increase in its refractivity. This phenomenon is the basis of quellung reaction.
Use in vaccination
Vaccination using capsular material is effective against some organisms (e.g., H. influenzae type b,[25][26] S. pneumoniae, and N. meningitidis[27]). However, polysaccharides are not highly antigenic, especially in children, so many capsular vaccines contain polysaccharides conjugated with protein carriers, such as the tetanus toxoid or diphtheria toxoid. This stimulates a much more robust immune response.[28]
See also
- Bacterial cell structure
- Quellung reaction, a method to visualize capsule under a microscope
References
- ↑ Peterson JW (1996). Bacterial Pathogenesis. Medical Microbiology. University of Texas Medical Branch at Galveston. ISBN 9780963117212. PMID 21413346. Retrieved 17 January 2018.
- ↑ Gao S, Lewis GD, Ashokkumar M, Hemar Y (January 2014). "Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of growth phase and capsule properties of the bacteria". Ultrasonics Sonochemistry. 21 (1): 446–53. doi:10.1016/j.ultsonch.2013.06.006. PMID 23835398.
- ↑ Hathaway LJ, Grandgirard D, Valente LG, Täuber MG, Leib SL (March 2016). "Streptococcus pneumoniae capsule determines disease severity in experimental pneumococcal meningitis". Open Biology. 6 (3): 150269. doi:10.1098/rsob.150269. PMC 4821241. PMID 27009189.
- ↑ "bacterial capsule" at Dorland's Medical Dictionary
- ↑ "Basteria: Capsules and Slime Layers". Encyclopædia Britannica. Archived from the original on 2013-03-08.
- ↑ Daffé M, Etienne G (1999). "The capsule of Mycobacterium tuberculosis and its implications for pathogenicity". Tubercle and Lung Disease. 79 (3): 153–69. doi:10.1054/tuld.1998.0200. PMID 10656114.
- ↑ Lindberg AA (November 1999). "Polyosides (encapsulated bacteria)". Comptes Rendus de l'Académie des Sciences, Série III. 322 (11): 925–32. Bibcode:1999CRASG.322..925L. doi:10.1016/s0764-4469(00)87188-7. PMID 10646085.
- ↑ "Meningococcal meningitis". Textbookofbacteriology.net. Archived from the original on 2014-02-09. Retrieved 2014-01-22.
- ↑ Ganesh K, Allam M, Wolter N, Bratcher HB, Harrison OB, Lucidarme J, et al. (February 2017). "Molecular characterization of invasive capsule null Neisseria meningitidis in South Africa". BMC Microbiology. 17 (1): 40. doi:10.1186/s12866-017-0942-5. PMC 5320719. PMID 28222677.
- ↑ Harrison OB, Claus H, Jiang Y, Bennett JS, Bratcher HB, Jolley KA, et al. (April 2013). "Description and nomenclature of Neisseria meningitidis capsule locus". Emerging Infectious Diseases. 19 (4): 566–73. doi:10.3201/eid1904.111799. PMC 3647402. PMID 23628376.
- ↑ Yoshida K, Matsumoto T, Tateda K, Uchida K, Tsujimoto S, Yamaguchi K (November 2000). "Role of bacterial capsule in local and systemic inflammatory responses of mice during pulmonary infection with Klebsiella pneumoniae". Journal of Medical Microbiology. 49 (11): 1003–10. doi:10.1099/0022-1317-49-11-1003. PMID 11073154.
- ↑ Dorman MJ, Feltwell T, Goulding DA, Parkhill J, Short FL (November 2018). "Klebsiella pneumoniae Defined by density-TraDISort". mBio. 9 (6). doi:10.1128/mBio.01863-18. PMC 6247091. PMID 30459193.
- ↑ Schembri MA, Blom J, Krogfelt KA, Klemm P (August 2005). "Capsule and fimbria interaction in Klebsiella pneumoniae". Infection and Immunity. 73 (8): 4626–33. doi:10.1128/IAI.73.8.4626-4633.2005. PMC 1201234. PMID 16040975.
- ↑ Schouls L, van der Heide H, Witteveen S, Zomer B, van der Ende A, Burger M, Schot C (February 2008). "Two variants among Haemophilus influenzae serotype b strains with distinct bcs4, hcsA and hcsB genes display differences in expression of the polysaccharide capsule". BMC Microbiology. 8 (1): 35. doi:10.1186/1471-2180-8-35. PMC 2267795. PMID 18298818.
- ↑ Deretic V, Dikshit R, Konyecsni WM, Chakrabarty AM, Misra TK (March 1989). "The algR gene, which regulates mucoidy in Pseudomonas aeruginosa, belongs to a class of environmentally responsive genes". Journal of Bacteriology. 171 (3): 1278–83. doi:10.1128/jb.171.3.1278-1283.1989. PMC 209741. PMID 2493441.
- ↑ Gibson DL, White AP, Snyder SD, Martin S, Heiss C, Azadi P, et al. (November 2006). "Salmonella produces an O-antigen capsule regulated by AgfD and important for environmental persistence". Journal of Bacteriology. 188 (22): 7722–30. doi:10.1128/JB.00809-06. PMC 1636306. PMID 17079680.
- ↑ Kenyon, Johanna J.; Hall, Ruth M. (2013-04-16). de Crécy-Lagard, Valerie (ed.). "Variation in the Complex Carbohydrate Biosynthesis Loci of Acinetobacter baumannii Genomes". PLOS ONE. 8 (4): e62160. Bibcode:2013PLoSO...862160K. doi:10.1371/journal.pone.0062160. ISSN 1932-6203. PMC 3628348. PMID 23614028.
- ↑ Singh, Jennifer K.; Adams, Felise G.; Brown, Melissa H. (2019-01-09). "Diversity and Function of Capsular Polysaccharide in Acinetobacter baumannii". Frontiers in Microbiology. 9: 3301. doi:10.3389/fmicb.2018.03301. ISSN 1664-302X. PMC 6333632. PMID 30687280.
- ↑ Hamaguchi S, Zafar MA, Cammer M, Weiser JN (March 2018). "Capsule Prolongs Survival of Streptococcus pneumoniae during Starvation". Infection and Immunity. 86 (3). doi:10.1128/IAI.00802-17. PMC 5820961. PMID 29311231.
- ↑ Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS (February 2010). "The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms". Infection and Immunity. 78 (2): 704–15. doi:10.1128/IAI.00881-09. PMC 2812187. PMID 19948837.
- ↑ O'Meara TR, Alspaugh JA (July 2012). "The Cryptococcus neoformans capsule: a sword and a shield". Clinical Microbiology Reviews. 25 (3): 387–408. doi:10.1128/CMR.00001-12. PMC 3416491. PMID 22763631.
- ↑ Gates MA, Thorkildson P, Kozel TR (April 2004). "Molecular architecture of the Cryptococcus neoformans capsule". Molecular Microbiology. 52 (1): 13–24. doi:10.1111/j.1365-2958.2003.03957.x. PMID 15049807.
- ↑ Casadevall A, Coelho C, Cordero RJ, Dragotakes Q, Jung E, Vij R, Wear MP (December 2019). "Cryptococcus neoformans". Virulence. 10 (1): 822–831. doi:10.1080/21505594.2018.1431087. PMC 6779390. PMID 29436899.
- ↑ Rudolph K (1996). "Chapter 3: Pseudomonas synringae pathovars". In Singh RP, Kohmoto K, Singh US (eds.). Pathogenesis & Host Specificity in Plant Diseases. Vol. 1: Prokaryotes (1st ed.). Amsterdam: Elsevier Science. ISBN 978-0-08-098473-5.
- ↑ Satola SW, Collins JT, Napier R, Farley MM (October 2007). "Capsule gene analysis of invasive Haemophilus influenzae: accuracy of serotyping and prevalence of IS1016 among nontypeable isolates". Journal of Clinical Microbiology. 45 (10): 3230–8. doi:10.1128/JCM.00794-07. PMC 2045354. PMID 17699642.
- ↑ Watts SC, Holt KE (June 2019). "In Silico Serotyping of the Haemophilus influenzae Capsule Locus". Journal of Clinical Microbiology. 57 (6). doi:10.1128/JCM.00190-19. PMC 6535587. PMID 30944197.
- ↑ Tzeng YL, Thomas J, Stephens DS (September 2016). "Regulation of capsule in Neisseria meningitidis". Critical Reviews in Microbiology. 42 (5): 759–72. doi:10.3109/1040841X.2015.1022507. PMC 4893341. PMID 26089023.
- ↑ Goldblatt D (January 2000). "Conjugate vaccines". Clinical and Experimental Immunology. 119 (1): 1–3. doi:10.1046/j.1365-2249.2000.01109.x. PMC 1905528. PMID 10671089.