Clavibacter michiganensis
| Clavibacter michiganensis | |
|---|---|
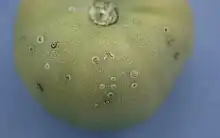 | |
| Symptoms of Clavibacter michiganensis on tomato | |
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Binomial name | |
| Clavibacter michiganensis corrig. (Smith 1910) Davis et al. 1984 | |
| Subspecies[1] | |
| |
| Synonyms | |
| |
Clavibacter michiganensis is an aerobic non-sporulating Gram-positive plant pathogenic actinomycete that currently constitutes the only species within the genus Clavibacter. The other former Clavibacter species have been reclassified to genera Leifsonia, Rathayibacter, and Curtobacterium. Clavibacter michiganensis has nine subspecies. Clavibacter michiganensis subsp. michiganensis and Clavibacter michiganensis subsp. sepedonicus cause substantial economic losses worldwide by damaging tomatoes and potatoes; Clavibacter michiganensis subsp. insidiosus causes damage to alfalfa, .[2]
Context
Clavibacter, also known as Ring Rot, is an unusual genus of phytopathogenic bacteria in that it is gram-positive and does not have a type three secretion system. All Clavibacter species and subspecies have a type B2γ cell wall crosslinked at a diaminobutyrate residue. Clavibacter is an aerobic bacterium with a coryneform morphology. There is no mycelium and no spores are produced.[3]
Clavibacter michiganensis infects the primary host in one of three ways: wounds, hydathodes, or by contaminated seed. If the bacteria reach a suitable quorum, the result is a systemic vascular infection. In the first stages of invasion, C. michiganensis resides as a biotrophic pathogen in the xylem vessels.[3]
Clavibacter has a complex history of taxonomical names. For a long time, there was only one recognized species within the genus Clavibacter. There are nine subspecies within the michiganensis species. Recently, some strains have been reclassified into other genera. This complex history stems from the difficulty in characterizing bacteria. Unlike fungi, the morphology of bacteria is not very sufficient for taxonomical purposes. To this end, strains of a phytopathogenic bacteria, called pathovars, are distinguished by cultural (selective media), physiological, biochemical (e.g. secreted enzymes the chemical responses of the plant), or pathological characteristics (including the range of susceptible hosts).[4]
Recently, two strains of this bacteria – subsp. sepidonicum and subsp. michiganensis – have had their genomes sequenced and annotated. There is still much to discover about this pathogen-host interaction but now that the genome has been sequenced, the rate of discoveries will likely increase. One of the main goals pertaining to research of these bacterial genomes is to develop resistant varieties. Unfortunately, no resistant varieties have yet been found.[5]
Genetics
The species has a single chromosome.[3]
C. m. subsp. sepedonicus
C. m. subsp. sepedonicus is a high-profile alien plant pathogen of A2 Quarantine status affecting only potatoes. It causes a disease in potatoes known as 'ring rot' due to the way it rots vascular tissue inside potato tubers[6] It is present in parts of Europe but is under statutory control under ‘Council Directive 93/85/EEC' of 4 October 1993 on the control of potato ring rot. This means that if an outbreak occurs, the outbreak must be controlled and if possible the disease has to be eradicated. If necessary, prohibitions are put into place to prevent further spread.
A plant showing symptoms of ring rot should be reported to the local plant health authority.
Hosts and symptoms
The subsp. sepidonicus is an economically important pathogen because it affects only potato, which was the 12th highest ranking commodity in 2009, generating $44,128,413,000 globally.[7] Like all bacteria in the genus Clavibacter michiganesis subsp. sepidonicus causes a systemic vascular infection by invading the xylem vessels and multiplying there which sometimes leads to plugged xylem vessels. When diagnosing a subsp. sepidonicus infection in potato, look for discoloration of the vascular ring within the tuber that has been described as "glassy" or "water-soaked" with the ooze inside having a "cheese-like consistency".[8][9]
Symptoms of potato 'ring rot' are yellowing of the leaf margins which later turn brown and look like they are burned. Tubers rot from the inside, sometimes progressing to leave hollow shells. Rotting of the tubers is the more common symptom. Infected land cannot be used again for susceptible crops for several years. Among others, United States, Canada, many EU states and Middle Eastern countries have not yet been able to eradicate this pathogen.
Disease cycle
The causal agent of Ring Rot of Potato overwinters many different ways. The bacteria survives in infected tubers in both storage and in the field. Diseased tubers then infect newly planted tubers. The bacterium also may be foundas dried slime on machinery or containers. For instance, if a knife cuts into an infected tuber, the next 20 tubers that the knife cuts have a high risk of becoming infected.[10] The bacteria enters the host through wounds and invades the xylem where it multiplies via binary fission. If colonization is successful, the bacteria may plug the xylem vessels. In advanced stages of infection, the bacteria will move out of the vessels and break down the surrounding parenchyma tissue before moving into new vessels. The bacteria may also invade the roots and cause them to deteriorate.[10]
C. sepidonicus spreads by contaminated soil, surfaces, infected seed, wash waters, infected potato waste, etc. It can survive on warehouse walls, boxes, bags etc. On machinery in dry conditions, it can survive at least a month – sometimes in the form of dried bacterial ooze. It is also able to overwinter in soil in association with plant debris. C. sepidonicus will only survive in the soil as long as the host tissue in which it resides persists and resists decomposition by saprophytic microorganisms in the soil. This poor ability to compete as a saprophyte in the absence of a susceptible host makes Clavibacter sp. known as soil invaders as opposed to soil inhabitants.[10]
Environment
North, Northwest and Central Europe have favorable climates for virulence. The disease multiplies rapidly and survives longer in cooler environments around 21 °C. At favorable conditions, the pathogen can survive 63 months in infected potato stems and 18 months in burlap sacks.[11]
Management
In the UK, DEFRA Plant Health and Seed Inspectors (PHSI) and SEERAD carry out annual survey work on ware and seed potatoes. Samples are sent to the Fera Science which was formerly known as The Central Science Laboratory (for England and Wales) and to Scottish Agricultural Science Agency (SASA) (for Scotland) for latent infection testing (infected but not showing symptoms). Infected crops once identified are intercepted, impounded, and destroyed. additional text. In the EU, quarantine facilities and licences are required to obtain, hold, and/or work with the bacteria and in the UK, Department of Trade and Industry (DTI) export licences are required to export it to countries outside of the EU whether through a third party country or not. The last known outbreak in the UK was in August 2004.[12]
There are no chemicals to treat ring rot of potato. There are no resistant varieties either. If a tuber tests positive for Clavibacter michiganensis subsp. sepidonicus, proper authorities should be immediately contacted as this is a quarantine disease in the United States as well Europe.[8]
Subsp. sepidonicus presents a danger of long-distance dispersal due to its ability to survive in seeds.[13]
C. m. subsp. michiganensis
C. m. subsp. michiganensis is the causative agent of bacterial wilt and canker of tomato (Lycopersicon esculentum).[3]
Hosts and symptoms
When the infection occurs in an early stage of the tomato plant there may be wilting on leaves because Clavibacter michiganensis subsp. michiganensis enter the plant by wounds, including root wounds, and if the bacterium gets to the xylem then a systemic infection is likely that may plug the xylem vessels. The wilting may only show on one side of the leaf and may recover during cooler periods.[14] The entire system of xylem within the plant allows the bacteria to form titers of up to 109 bacteria per gram of plant tissue. Wilting may eventually spread to all leaves and these leaves, along with their petioles, may also show distorted, curled growth. One way to diagnose a severe vascular infection is to pinch the stem. If the epidermis and outer layer of the cortex separate from the inner stem then there is severe vascular infection. These exposed parts will have a soapy feel.[14] Canker lesions, though rare, may develop on the stem. These cankers are necrotic regions where the epidermis is gone. As the bacteria continues its colonization, the canker will deepen and expand. In terms of fruit development, tomatoes may fail to develop altogether or may look marbled because they are ripening unevenly.[14]
If infection occurs at a late stage of plant development, plants are able to survive and generate fruits. However, the plant may appear stressed rather than wilted and may develop white interveinal areas that will develop into brown necrotic tissue. Often the seeds are infected as well.[2]
Superficial infections increase the risk of epidemics.[14] They occur when the bacteria multiply on the epidermis of the host, enter through stomata, or enter through a very shallow wound that does not allow the pathogen to reach the xylem tissue. The host may look like it was rubbed with cornmeal or coarse flour but it is actually a series of blisters that me be raised or sunken and appear white to pale orange.[14] The most common leaf symptom is a dark brown spot surrounded by a sort of orange-like area on the edge of the leaf. Fruits may develop "bird's eye" spotting, which are pale green to white raised pustules that have a brown center and chlorotic halo.[14] Pictures of these symptoms are available at the cited reference.
However, latent infections are common.[3]
The Clavibacter michiganensis subsp. michiganensis wild type strain NCPPB382 carries two plasmids associated with virulence: pCM1 and pCM2. The avirulent strain, CMM100, does not contain these plasmids. Strains that carried one of the two plasmids were found to be virulent but wilting symptoms were delayed. The virulent and avirulent strains produced the same amount of exopolysaccharides, suggesting that EPS does not play a significant role in pathogenicity.[15]
Disease cycle
The causal agent of bacterial wilt and canker of tomato survives in or on seeds for up to 8 months[14] but occasionally also in plant refuse in the soil. The pathogen can be spread long distances because of its association with seeds.[16] The risk of spreading the bacteria to healthy tomato plants is greatest during transplanting, tying, and suckering or any time when the host may be wounded. Once the bacteria enters the plant through a wound, it will move and multiply primarily in the xylem vessels. Once established, the bacteria may move into the phloem, pith, and cortex.[10] Infection can result in either systemic or superficial disease. Systemic infections appear in 3–6 weeks and the risk of secondary infection goes up with water-splashing.[14] The common occurrence of latent infections – presence of the pathogen within the host yet the host shows no symptoms – makes this pathogen especially dangerous.[17]
However, the assumption that C. michiganensis does not overwinter in the soil is not without controversy. The genome of C. michiganesis has recently been sequenced and new theories will surely arise once more work has been completed. What is known is that Cmm can use hydrolysis products as carbon and energy sources by means of a number of ATP-binding cassette transporters and α- and β-glucosidases.[18] This suggests that Cmm can survive in the soil as long as there is decaying host material present. It has also been determined that the genome of subsp. michiganensis does not have genes that encode for nitrate and nitrate reductases. This means that the bacteria depends on previously reduced nitrogen compounds or amino acids for its nitrogen source.[18] Also lacking in the Cmm genome are genes for assimilatory sulfate reduction, which is associated with an auxotrophy for methionine – one of two amino acids that contain sulphur.[18]
Cmm has a pathogenicity island (PI) that is encoded in the chromosome and is probably associated with colonization and plant defense evasion or suppression. This island has been subdivided into two subregions: chp and tomA. Serine proteases of the families S1A, Ppa, and PpA-E are encoded in the chp subregion as well as subtilase SbtA.[3]
Environment
Warm temperature in the range of 23–28 °C (73–82 °F) and the high relative humidity (>80%) are optimal environments for Clavibacter michiganesis subsp. michiganesis, a tomato bacterial canker symptom development.[19] In humid or wet weather, slimy masses of bacteria ooze through the cracks to the surface of the stem, from which they are spread to leaves and fruits and cause secondary infections[10] Infected host plants will show severe symptoms on hot days when there is a high transpiration rate since the bacteria may plug the xylem vessels.
Management
The best way to control a disease is an use of healthy seeds that have already been acid extracted. In addition, using chemical treatments such as copper hydroxide[20] or streptomycin in the seed bed,[10] removing or isolating diseased crops can be helpful to reduce the rate of infection.[12]
C. m. subsp. nebraskensis
C. m. subsp. nebraskensis causes wilt and blight in maize,[2][3] called Goss's wilt.[21]
Genetics
An annotated nucleotide sequence was expected to be available soon after 2011. The single chromosome is of 3.06megabase (of which the GC-content is 73.0), mostly collinear, and contains 2 rRNA operons, and 45 tRNAs. As of 2011 when a partial annotation was available, it appeared to contain 50 pseudogenes and no insertion elements. A chloride channel is suspected to be a virulence factor.[3]
Other subspecies
Other subspecies include:[1]
- Clavibacter michiganensis subsp. californiensis
- Clavibacter michiganensis subsp. capsici
- Clavibacter michiganensis subsp. chilensis
- Clavibacter michiganensis subsp. michiganensis
- Clavibacter michiganensis subsp. phaseoli
- Clavibacter michiganensis subsp. tessellarius – induces leaf freckles and leaf spots in wheat[2][3]
- Clavibacter michiganensis subsp. insidiosus – causes wilting and stunting in alfalfa.[2]
- Clavibacter michiganensis subsp. sepedonicus
References
- 1 2 "Clavibacter michiganensis". NCBI taxonomy. Bethesda, MD: National Center for Biotechnology Information. Retrieved 13 December 2018.
- 1 2 3 4 5 Gartemann et al. "Clavibacter michiganensis subsp. michiganensis: first steps in the understanding of virulence of a Gram-positive phytopathogenic bacterium", Journal of Biotechnology, Germany, 16 July 2003. Retrieved on 2011-10-26.
- 1 2 3 4 5 6 7 8 9 Eichenlaub, Rudolf; Gartemann, Karl-Heinz (8 September 2011). "The Clavibacter michiganensis Subspecies: Molecular Investigation of Gram-Positive Bacterial Plant Pathogens". Annual Review of Phytopathology. Annual Reviews. 49 (1): 445–464. doi:10.1146/annurev-phyto-072910-095258. ISSN 0066-4286. PMID 21438679. S2CID 207707582.
- ↑ Eichenlaub R, Gartemann K-H, Burger A. 2006 Clavibacter michiganensis, a group of Gram positive phytopathogenic bacteria. In "Plant-Associated Bacteria", ed. SS Gnanamanickam, pp. 385–422. Dordrecht: Springer
- ↑ Coaker GL, Willard B, Kinter M, Stockinger EJ, Francis DM. 2004. Proteomic analysis of resistance mediated by Rem 2.0 and Rem 5.1, two loci controlling resistance to bacterial canker of tomato. Mol. Plant-Microbe Interact. 17: 1019–28
- ↑ Manzer F, Genereux H.1981.Ring Rot. In Compendium of Potato Disease, ed. WJ Hobson, pp. 31–32.St Paul, MN:Am Phytopathol.Soc.Press
- ↑ Food and Agriculture Organization of the United Nations. http://faostat.fao.org/site/339/default.aspx
- 1 2 (2009). Bacterial rots of potato tubers. Retrieved from United Kingdom Food and Environment Research Agency website: http://www.fera.defra.gov.uk/plants/publications/documents/factsheets/bacterialRotsPotato.pdf Archived 8 November 2011 at the Wayback Machine
- ↑ Evtushenko LI, Takeuchi M. 2006. The family Microbacteriaceae. In "The Prokaryotes", 3rd edition, ed. M Dworkin, S Falkow, E Rosenburg, KH Schleifer, E Stackenbrandt, 3:1020–99. New York: Springer
- 1 2 3 4 5 6 Agrios, George N (2005). Plant Pathology, Burlington, MA: Elsevier Academic Press. ISBN 978-0-12-044565-3.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 23 April 2011. Retrieved 26 October 2011.
{{cite web}}: CS1 maint: archived copy as title (link), CABI and EPPO for the EU. "Clavibacter michiganensis subsp. sepedonicus", EPPO quarantine pest. Retrieved on 2011-10-26. - 1 2 "Archived copy" (PDF). Archived from the original (PDF) on 17 May 2011. Retrieved 25 October 2011.
{{cite web}}: CS1 maint: archived copy as title (link), CABI and EPPO for the EU. "Clavibacter michiganensis subsp. michiganensis", EPPO quarantine pest. Retrieved on 2011-10-26. - ↑ Bugbee WM, Gudmestad NC. 1988. The recovery of Corneybacterium sepidonicum from sugarbeet seed. Phytopathology 78:205-8
- 1 2 3 4 5 6 7 8 Elphinstone J, O'Neill T. Bacterial wilt and canker of tomato(Clavibacter michiganensis subsp.michiganensis).Tomato factsheet.2010. Horticulture Development Company
- ↑ Meletzus D, Bermpohl A, Drier J, Eichenlaub R. 1993. Evidence for plasmid-encoded virulence factors in the phytopathogenic bacterium Clavibacter michiganensis subsp. michiganensis NCPPB382. J. Bacteriol. 175:2131-36.
- ↑ Tsiantos J.1987 Transmission of the bacterium Corneybacterium michiganense pv. michiganense by seeds. J. Phytopathol.119:142-46
- ↑ Gitaitis RD, Beaver RW, Voloudakis AE.1991. Detection of Clavibacter michiganensis subsp. michiganensis in symptomless tomato transplants. Plant Dis.75:834-38
- 1 2 3 Gartemann KH, Abt B, Bekel T, Burger A, Engemann J, et al. 2008. The genome sequence of the tomato-pathogenic actinomycete Clavibacter michiganensissubsp michiganensis NCPPB382 reveals a large island involved in pathogenicity. J.Bacteriol.190:2138-49
- ↑ Xu et al. "Colonization of Tomato Seedlings by Bioluminescent Clavibacter michiganensis subsp. michiganesis under Different Humidity Regimes", Phytopathology, Ohio. Retrieved on 2011-11-16.
- ↑ , Werner at al. "Limiting Populations and Spread of Clavibacter michiganensis subsp. michiganensis on Seedling Tomatoes in the Greenhouse", Plant Disease, East Lansing, May 2002. Retrieved on 2011-10-26.
- ↑ "Goss's Wilt of Corn". Crop Protection Network. Retrieved 15 July 2021.