Cryptophycin
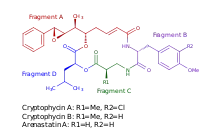
Cryptophycins are a family of macrolide molecules that are potent cytotoxins and have been studied for potential antiproliferative properties useful in developing chemotherapy. They are members of the depsipeptide family.
History
Cryptophycins were originally discovered in 1990 in cyanobacteria of the genus Nostoc.[1] Cryptophycins were patented as antifungal agents with an unknown mechanism of action and subsequently identified as microtubule inhibitors.[2] Closely related molecules were reported in the marine sponge Dysidea arenaria, which were first given the name arenastatins.[3] However, since cyanobacteria are common symbionts of sponges, it has been suggested that bacteria may be the true origin in cases where sponge and bacterial metabolites closely resemble one another.[4] Nevertheless, study of the structure-activity relationships between the two subgroups of molecules led to improved understanding of their cytotoxic effects.[5]: 230
Mechanism of action
Cryptophycins are potent microtubule inhibitors, with a mechanism of action similar to that of vinca alkaloids.[2][6][7] Treatment of cells with cryptophycins depletes microtubules through interaction with tubulin, thereby preventing cell division.[8] Cryptophycins are capable of inducing apoptosis,[9] possibly through other mechanisms in addition to that mediated by microtubule inhibition.[10]
Clinical studies
Members of the cryptophycin family have been studied as anti-tumor agents. Cryptophycin-52, a synthetic analog of natural product cryptophycins also known as LY355703,[11] reached phase II clinical trials but was withdrawn due to side effects.[12]
Synthesis
Cryptophycins were first isolated from cyanobacteria but have subsequently been produced by chemical synthesis.[13][14] Chemoenzymatic syntheses have also been reported.[15][16]
References
- ↑ Schwartz, Robert E.; Hirsch, Charles F.; Sesin, David F.; Flor, James E.; Chartrain, Michel; Fromtling, Robert E.; Harris, Guy H.; Salvatore, Michael J.; Liesch, Jerrold M.; Yudin, Katherine (April 1990). "Pharmaceuticals from cultured algae". Journal of Industrial Microbiology. 5 (2–3): 113–123. doi:10.1007/BF01573860. S2CID 34480729.
- 1 2 Smith, CD; Zhang, X; Mooberry, SL; Patterson, GM; Moore, RE (15 July 1994). "Cryptophycin: a new antimicrotubule agent active against drug-resistant cells". Cancer Research. 54 (14): 3779–84. PMID 7913408.
- ↑ KOBAYASHI, Motomasa; KUROSU, Michio; OHYABU, Naoki; WANG, Weiqi; FUJII, Satoshi; KITAGAWA, Isao (1994). "The Absolute Stereostructure of Arenastatin A, a Potent Cytotoxic Depsipeptide from the Okinawan Marine Sponge Dysidea arenaria". Chemical & Pharmaceutical Bulletin. 42 (10): 2196–2198. doi:10.1248/cpb.42.2196.
- ↑ Piel, Jörn (2004-01-01). "Metabolites from symbiotic bacteriaThis review is dedicated to Professor Axel Zeeck on the occasion of his 65th birthday". Natural Product Reports. 21 (4): 519–38. doi:10.1039/b310175b. PMID 15282634.
- ↑ Cragg, edited by Gordon M.; Kingston, David G.I.; Newman, David J. (2012). Anticancer agents from natural products (2nd ed.). Boca Raton, FL: CRC Press. ISBN 9781439813836.
{{cite book}}:|first1=has generic name (help) - ↑ Panda, D; DeLuca, K; Williams, D; Jordan, MA; Wilson, L (4 August 1998). "Antiproliferative mechanism of action of cryptophycin-52: kinetic stabilization of microtubule dynamics by high-affinity binding to microtubule ends". Proceedings of the National Academy of Sciences of the United States of America. 95 (16): 9313–8. Bibcode:1998PNAS...95.9313P. doi:10.1073/pnas.95.16.9313. PMC 21335. PMID 9689077.
- ↑ Panda, D; Himes, RH; Moore, RE; Wilson, L; Jordan, MA (21 October 1997). "Mechanism of action of the unusually potent microtubule inhibitor cryptophycin 1". Biochemistry. 36 (42): 12948–53. doi:10.1021/bi971302p. PMID 9335554.
- ↑ Zhang, X. (15 March 1996). "Mechanism of Action of Cryptophycin". Journal of Biological Chemistry. 271 (11): 6192–6198. doi:10.1074/jbc.271.11.6192. PMID 8626409.
- ↑ Mooberry, SL; Busquets, L; Tien, G (4 November 1997). "Induction of apoptosis by cryptophycin 1, a new antimicrotubule agent". International Journal of Cancer. 73 (3): 440–8. doi:10.1002/(sici)1097-0215(19971104)73:3<440::aid-ijc20>3.3.co;2-x. PMID 9359493.
- ↑ Drew, L; Fine, RL; Do, TN; Douglas, GP; Petrylak, DP (December 2002). "The novel antimicrotubule agent cryptophycin 52 (LY355703) induces apoptosis via multiple pathways in human prostate cancer cells". Clinical Cancer Research. 8 (12): 3922–32. PMID 12473608.
- ↑ Trimurtulu, Golakoti; Ohtani, Ikuko; Patterson, Gregory M. L.; Moore, Richard E.; Corbett, Thomas H.; Valeriote, Frederick A.; Demchik, Lisa (June 1994). "Total Structures of Cryptophycins, Potent Antitumor Depsipeptides from the Blue-Green Alga Nostoc sp. Strain GSV 224". Journal of the American Chemical Society. 116 (11): 4729–4737. doi:10.1021/ja00090a020.
- ↑ Field, Jessica J.; Kanakkanthara, Arun; Miller, John H. (September 2014). "Microtubule-targeting agents are clinically successful due to both mitotic and interphase impairment of microtubule function". Bioorganic & Medicinal Chemistry. 22 (18): 5050–5059. doi:10.1016/j.bmc.2014.02.035. PMID 24650703.
- ↑ Bolduc, KL; Larsen, SD; Sherman, DH (22 May 2012). "Efficient, divergent synthesis of cryptophycin unit A analogues". Chemical Communications (Cambridge, England). 48 (51): 6414. doi:10.1039/c2cc32417b. PMC 3494784. PMID 22617820.
- ↑ Weiß, C; Bogner, T; Sammet, B; Sewald, N (2012). "Total synthesis and biological evaluation of fluorinated cryptophycins". Beilstein Journal of Organic Chemistry. 8: 2060–6. doi:10.3762/bjoc.8.231. PMC 3511040. PMID 23209540.
- ↑ Magarvey, NA; Beck, ZQ; Golakoti, T; Ding, Y; Huber, U; Hemscheidt, TK; Abelson, D; Moore, RE; Sherman, DH (15 December 2006). "Biosynthetic characterization and chemoenzymatic assembly of the cryptophycins. Potent anticancer agents from cyanobionts". ACS Chemical Biology. 1 (12): 766–79. doi:10.1021/cb6004307. PMID 17240975.
- ↑ Ding, Y; Rath, CM; Bolduc, KL; Håkansson, K; Sherman, DH (21 September 2011). "Chemoenzymatic synthesis of cryptophycin anticancer agents by an ester bond-forming non-ribosomal peptide synthetase module". Journal of the American Chemical Society. 133 (37): 14492–5. doi:10.1021/ja204716f. PMC 3174474. PMID 21823639.