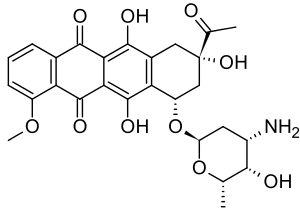
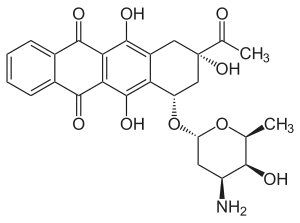
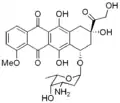
Daunosamine
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Linear:(3S,4S,5S)-3-amino-4,5-dihydroxyhexanal Pyranose: (3S,4S,5S)-4-amino-6-methyl-tetrahydropyran-2,5-diol | |||
| Other names
3-Amino-2,3,6-trideoxy-L-lyxo-hexose | |||
| Identifiers | |||
CAS Number |
| ||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
C6H13NO3 | ||
| Molar mass | 147.174 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Daunosamine is a deoxy sugar and amino sugar of the hexosamine class.[1]
Daunosamine is a component of the anthracycline class of antineoplastics, linked to a derivative of naphthacene. It is a component of birch juice.
The compound is soluble in water and responds with polymers like cellulose and lignin if it is in excess, so collection of birch juice is very helpful for the birch tree.
The dnrQ gene is required for the synthesis of daunosamine.[2]
References
- ↑ Grethe, Guenter; Mitt, Toomas; Williams, Thomas H; Uskokovic, Milan R (1983). "Synthesis of daunosamine". The Journal of Organic Chemistry. 48 (26): 5309–5315. doi:10.1021/jo00174a028.
- ↑ Otten, S. L; Liu, X; Ferguson, J; Hutchinson, C. R (1995). "Cloning and characterization of the Streptomyces peucetius dnrQS genes encoding a daunosamine biosynthesis enzyme and a glycosyl transferase involved in daunorubicin biosynthesis". Journal of Bacteriology. 177 (22): 6688–92. doi:10.1128/jb.177.22.6688-6692.1995. PMC 177529. PMID 7592454.
External links
- Daunosamine at the US National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.