Diet and cancer
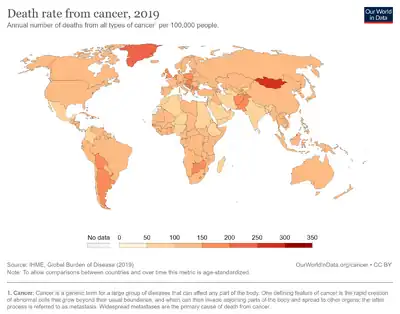
Dietary factors are recognized as having a significant effect on the risk of cancers, with different dietary elements both increasing and reducing risk. Diet and obesity may be related to up to 30–35% of cancer deaths,[1] while physical inactivity appears to be related to 7% risk of cancer occurrence.[2] One review in 2011 suggested that total caloric intake influences cancer incidence and possibly progression.[3]
While many dietary recommendations have been proposed to reduce the risk of cancer, few have significant supporting scientific evidence.[3] Obesity and drinking alcohol have been correlated with the incidence and progression of some cancers.[3] Lowering the drinking of beverages sweetened with sugar is recommended as a measure to address obesity.[4] A diet low in fruits and vegetables and high in red meat has been implicated but not confirmed,[5] and the effect may be small for well-nourished people who maintain a healthy weight.[3]
Some specific foods are linked to specific cancers. Studies have linked eating red or processed meat to an increased risk of breast cancer, colon cancer,[4] prostate cancer,[6] and pancreatic cancer, which may be partially explained by the presence of carcinogens in foods cooked at high temperatures.[7][8] Aflatoxin B1, a frequent food contaminant, increases risk of liver cancer,[9] while drinking coffee is associated with a reduced risk.[10] Betel nut chewing causes oral cancer.[9] Stomach cancer is more common in Japan due to its high-salt diet.[9][11] Immigrant communities tend to develop the risk of their new country, often within one generation, suggesting a substantial link between diet and cancer.[12]
Dietary recommendations for cancer prevention typically include weight management and eating "mainly vegetables, fruit, whole grains and fish, and a reduced intake of red meat, animal fat, and refined sugar."[3]
Types of diet

Restrictive diets
A number of diets and diet-based regimes are claimed to be useful against cancer. Popular types of "anti-cancer" diets include the Breuss diet, Gerson therapy, the Budwig protocol and the macrobiotic diet. None of these diets has been found to be effective, and some of them have been found to be harmful.[13]
Dietary patterns
Nutritional epidemiologists use multivariate statistics, such as principal components analysis and factor analysis, to measure how patterns of dietary behavior influence the risk of developing cancer.[14] (The most well-studied dietary pattern is the mediterranean diet.) Based on their dietary pattern score, epidemiologists categorize people into quantiles. To estimate the influence of dietary behavior on risk of cancer, they measure the association between quantiles and the distribution of cancer prevalence (in case-control studies) and cancer incidence (in longitudinal studies). They usually include other variables in their statistical model to account for the other differences between people with and without cancer (confounders). For breast cancer, there is a replicated trend for women with a more "prudent or healthy" diet, i.e. higher in fruits and vegetables, to have a lower risk of cancer.[15] A "drinker dietary pattern" is also associated with higher breast cancer risk, while the association is inconsistent between a more westernized diet and elevated risk of breast cancer. Pickled foods are linked with cancer.
Dietary components
Alcohol
Alcohol is associated with an increased risk of a number of cancers.[16] 3.6% of all cancer cases and 3.5% of cancer deaths worldwide are attributable to drinking of alcohol.[17] Breast cancer in women is linked with alcohol intake.[3][18] Alcohol also increases the risk of cancers of the mouth, esophagus, pharynx and larynx,[19] colorectal cancer,[20][21] liver cancer,[22] stomach[23] and ovaries.[24] The International Agency for Research on Cancer (Centre International de Recherche sur le Cancer) of the World Health Organization has classified alcohol as a Group 1 carcinogen. Its evaluation states, "There is sufficient evidence for the carcinogenicity of alcoholic beverages in humans. ... Alcoholic beverages are carcinogenic to humans (Group 1)."[25]
Processed and red meat
On October 26, 2015, the International Agency for Research on Cancer of the World Health Organization reported that eating processed meat (e.g., bacon, ham, hot dogs, sausages) or red meat was linked to some cancers and classed them as Group 1 (carcinogenic to humans) and Group 2a (probably carcinogenic to humans) carcinogens respectively.[26] There is some evidence that suggests that heme and nitrite are involved in the processes linking red and processed meat intake with colorectal cancer.[26] Heme is present in particular in red meat and nitrite is used as curing salt in many processed meats.
Fiber, fruits and vegetables
The evidence on the effect of dietary fiber on the risk of colon cancer is mixed with some types of evidence showing a benefit and others not.[4] While eating fruit and vegetables has a benefit, it has less benefit on reducing cancer than once thought.[4] Soy is rich in phytoestrogens. Phytoestrogens have weak estrogenic effects, but are naturally occurring compounds.[27]
Two 2020 meta-analyses found that a high fiber intake was associated with a lower risk of both premenopausal and postmenopausal breast cancers[28] and a higher survival rate in patients with breast cancer.[29]
A 2014 study found fruit but not vegetables protected against upper gastrointestinal tract cancer.[30] While fruit, vegetable and fiber protected against colorectal cancer and fiber protected against liver cancer.[30]
Flavonoids
Flavonoids (specifically flavonoids such as the catechins) are "the most common group of polyphenolic compounds in the human diet and are found ubiquitously in plants."[31] While some studies have suggested flavonoids may have a role in cancer prevention, others have been inconclusive or suggested they may be harmful.[32][33]
Mushrooms
According to Cancer Research UK, "there is currently no evidence that any type of mushroom or mushroom extract can prevent or cure cancer", although research into some species continues.[34]
Other
- According to the American Cancer Society, there is no conclusive evidence for an anticancer effect of consuming soy products.[35]
- Green tea consumption has no effect on cancer risk.[36][37][38]
- A 2016 meta-analysis showed that women and men who drank coffee had a lower risk of liver cancer.[10] An umbrella review of meta-analyses found that coffee was associated with a lower risk of liver and endometrial cancer.[39]
- A 2014 systematic review found, "no firm evidence that vitamin D supplementation affects cancer occurrence in predominantly elderly community-dwelling women."[40]
Mechanisms of action
Methionine metabolism
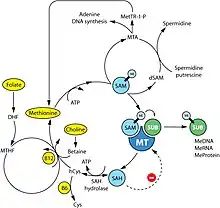
Although numerous cellular mechanisms are involved in food intake, many investigations over the past decades have pointed out defects in the methionine metabolic pathway as cause of carcinogenesis.[41][42] For instance, deficiencies of the main dietary sources of methyl donors, methionine and choline, lead to the formation of liver cancer in rodents.[43][44] Methionine is an essential amino acid that must be provided by dietary intake of proteins or methyl donors (choline and betaine found in beef, eggs and some vegetables). Assimilated methionine is transformed in S-adenosyl methionine (SAM) which is a key metabolite for polyamine synthesis, e.g. spermidine, and cysteine formation (see the figure on the right). Methionine breakdown products are also recycled back into methionine by homocysteine remethylation and methylthioadenosine (MTA) conversion (see the figure on the right). Vitamins B6, B12, folic acid and choline are essential cofactors for these reactions. SAM is the substrate for methylation reactions catalyzed by DNA, RNA and protein methyltransferases.
The products of these reactions are methylated DNA, RNA or proteins and S-adenosylhomocysteine (SAH). SAH has a negative feedback on its own production as an inhibitor of methyltransferase enzymes. Therefore, SAM:SAH ratio directly regulates cellular methylation, whereas levels of vitamins B6, B12, folic acid and choline regulates indirectly the methylation state via the methionine metabolism cycle.[45][46] A near ubiquitous feature of cancer is a maladaption of the methionine metabolic pathway in response to genetic or environmental conditions resulting in depletion of SAM and/or SAM-dependent methylation. Whether it is deficiency in enzymes such as methylthioadenosine phosphorylase, methionine-dependency of cancer cells, high levels of polyamine synthesis in cancer, or induction of cancer through a diet deprived of extrinsic methyl donors or enhanced in methylation inhibitors, tumor formation is strongly correlated with a decrease in levels of SAM in mice, rats and humans.[47][48]
According to a 2012 review, the effect of methionine restriction on cancer has yet to be studied directly in humans and "there is still insufficient knowledge to give reliable nutritional advice".[49]
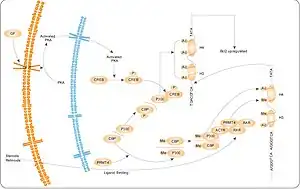
AMPK
AMPK is thought to be a major element or mechanism in cancer-related effects of diet. It modulates the activity of cellular survival signaling such as mTOR and Akt, leading to cell growth inhibition which is relevant to cancer growth. Targeting AMPK has become a novel strategy for cancer prevention and treatment.[50][51][52] Potential complementary or preventive options under investigation include periods of caloric restriction and AMPK agonists (typically mTOR inhibitors).[53][54][55][56][57][58] However, AMPK can also promote cancer in some settings.[50][55]
See also
- Alcohol and cancer
- Alcohol and breast cancer
- Acrylamide
- Bovine Meat and Milk Factors
- Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective
- List of ineffective cancer treatments
- List of topics characterized as pseudoscience
- Microplastics ingested through diet
- Zero waste supermarket
References
- ↑ Anand P, Kunnumakkara AB, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, et al. (September 2008). "Cancer is a preventable disease that requires major lifestyle changes". Pharmaceutical Research. 25 (9): 2097–2116. doi:10.1007/s11095-008-9661-9. PMC 2515569. PMID 18626751.
- ↑ Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. (June 2016). "Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults". JAMA Internal Medicine. 176 (6): 816–825. doi:10.1001/jamainternmed.2016.1548. PMC 5812009. PMID 27183032.
- 1 2 3 4 5 6 Wicki A, Hagmann J (9 September 2011). "Diet and cancer". Swiss Medical Weekly. 141: w13250. doi:10.4414/smw.2011.13250. PMID 21904992.
- 1 2 3 4 Stewart BW, Wild CP, eds. (2014). "Ch. 2: Cancer Etiology § 6 Diet, obesity and physical activity". World Cancer Report 2014. World Health Organization. pp. 124–33. ISBN 9789283204299.
- ↑ Key TJ (January 2011). "Fruit and vegetables and cancer risk". British Journal of Cancer. 104 (1): 6–11. doi:10.1038/sj.bjc.6606032. PMC 3039795. PMID 21119663.
- ↑ Joshi AD, Corral R, Catsburg C, Lewinger JP, Koo J, John EM, et al. (November 2012). "Red meat and poultry, cooking practices, genetic susceptibility and risk of prostate cancer: results from a multiethnic case-control study". Carcinogenesis. 33 (11): 2108–2118. doi:10.1093/carcin/bgs242. PMC 3584966. PMID 22822096.
- ↑ Zheng W, Lee SA (2009). "Well-done meat intake, heterocyclic amine exposure, and cancer risk". Nutrition and Cancer. 61 (4): 437–446. doi:10.1080/01635580802710741. PMC 2769029. PMID 19838915.
- ↑ Ferguson LR (February 2010). "Meat and cancer". Meat Science. 84 (2): 308–313. doi:10.1016/j.meatsci.2009.06.032. PMID 20374790.
- 1 2 3 Park S, Bae J, Nam BH, Yoo KY (2008). "Aetiology of cancer in Asia" (PDF). Asian Pacific Journal of Cancer Prevention. 9 (3): 371–380. PMID 18990005. Archived (PDF) from the original on 2022-12-06. Retrieved 2022-12-08.
- 1 2 Yu C, Cao Q, Chen P, Yang S, Deng M, Wang Y, Li L (December 2016). "An updated dose-response meta-analysis of coffee consumption and liver cancer risk". Scientific Reports. 6 (1): 37488. Bibcode:2016NatSR...637488Y. doi:10.1038/srep37488. PMC 5133591. PMID 27910873.
- ↑ Brenner H, Rothenbacher D, Arndt V (2009). Mukesh V (ed.). "Epidemiology of Stomach Cancer". Methods in Molecular Biology. 472: 467–477. doi:10.1007/978-1-60327-492-0_23. ISBN 9781603274913. PMC 2166976. PMID 19107449.
- ↑ Buell P, Dunn JE (May 1965). "Cancer mortality among Japanese Issei and Nisei of California". Cancer. 18 (5): 656–664. doi:10.1002/1097-0142(196505)18:5<656::AID-CNCR2820180515>3.0.CO;2-3. PMID 14278899.
- ↑ Hübner J, Marienfeld S, Abbenhardt C, Ulrich CM, Löser C (November 2012). "[How useful are diets against cancer?]". Deutsche Medizinische Wochenschrift. 137 (47): 2417–2422. doi:10.1055/s-0032-1327276. PMID 23152069.
- ↑ Edefonti V, Randi G, La Vecchia C, Ferraroni M, Decarli A (June 2009). "Dietary patterns and breast cancer: a review with focus on methodological issues". Nutrition Reviews. 67 (6): 297–314. doi:10.1111/j.1753-4887.2009.00203.x. PMID 19519672.
- ↑ Brennan SF, Cantwell MM, Cardwell CR, Velentzis LS, Woodside JV (May 2010). "Dietary patterns and breast cancer risk: a systematic review and meta-analysis". The American Journal of Clinical Nutrition. 91 (5): 1294–1302. doi:10.3945/ajcn.2009.28796. PMID 20219961.
- ↑ National Institute on Alcohol Abuse and Alcoholism (NIAAA) (July 1993). "Alcohol and Cancer". Alcohol Alert. 21: PH 345. Archived from the original on 2005-12-23.
- ↑ Boffetta P, Hashibe M, La Vecchia C, Zatonski W, Rehm J (August 2006). "The burden of cancer attributable to alcohol drinking". International Journal of Cancer. 119 (4): 884–887. doi:10.1002/ijc.21903. hdl:2434/22728. PMID 16557583. S2CID 14938863.
- ↑ Seitz HK, Pelucchi C, Bagnardi V, La Vecchia C (May–June 2012). "Epidemiology and pathophysiology of alcohol and breast cancer: Update 2012". Alcohol and Alcoholism. 47 (3): 204–212. doi:10.1093/alcalc/ags011. PMID 22459019.
- ↑ Marmot M, Atinmo T, Byers T, Chen J, Hirohata T, Jackson A, et al. (2007). "Ch. 4: Food and Drinks §8: Alcoholic drinks" (PDF). Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective (PDF). World Cancer Research Fund / American Institute for Cancer Research (AICR) Expert Reports. Vol. 2. Washington, DC: AICR. pp. 157–71. ISBN 9780972252225. Archived from the original (PDF) on 2016-05-07. Retrieved 2014-08-29.
- ↑ Su LJ, Arab L (2004). "Alcohol consumption and risk of colon cancer: evidence from the national health and nutrition examination survey I epidemiologic follow-up study". Nutrition and Cancer. 50 (2): 111–119. doi:10.1207/s15327914nc5002_1. PMID 15623458. S2CID 25461607.
- ↑ Cho E, Smith-Warner SA, Ritz J, van den Brandt PA, Colditz GA, Folsom AR, et al. (April 2004). "Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies". Annals of Internal Medicine. 140 (8): 603–613. doi:10.7326/0003-4819-140-8-200404200-00007. PMID 15096331. S2CID 37915731.
- ↑ Voigt MD (February 2005). "Alcohol in hepatocellular cancer". Clinics in Liver Disease. 9 (1): 151–169. doi:10.1016/j.cld.2004.10.003. PMID 15763234.
- ↑ Benedetti A, Parent ME, Siemiatycki J (2009). "Lifetime consumption of alcoholic beverages and risk of 13 types of cancer in men: results from a case-control study in Montreal". Cancer Detection and Prevention. 32 (5–6): 352–362. doi:10.1016/j.canep.2009.03.001. PMID 19588541.
- ↑ Bagnardi V, Blangiardo M, La Vecchia C, Corrao G (2001). "Alcohol consumption and the risk of cancer: a meta-analysis". Alcohol Research & Health. 25 (4): 263–270. PMC 6705703. PMID 11910703. Archived from the original on 2019-06-20. Retrieved 2022-12-08.
- ↑ Berrino F, Grant M, Griciute L, Holmberg B, McMichael AJ, Møller-Jensen O, et al. (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Alcohol Drinking) (1988). "Ch. 6: Summary of Data Reported and Evaluation §5: Evaluation" (PDF). Alcohol Drinking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 44. Lyon: International Agency for Research on Cancer (IARC): World Health Organization. pp. 258–9. ISBN 978-9283212447.
- 1 2 Staff (October 26, 2015). "World Health Organization - IARC Monographs evaluate consumption of red meat and processed meat" (PDF). International Agency for Research on Cancer. Archived (PDF) from the original on October 26, 2015. Retrieved October 26, 2015.
- ↑ Olsen, N, "Cancer and Food" Archived 2022-11-17 at the Wayback Machine BetterHealth.vic.gov.au
- ↑ Farvid MS, Spence ND, Holmes MD, Barnett JB (July 2020). "Fiber consumption and breast cancer incidence: A systematic review and meta-analysis of prospective studies". Cancer. 126 (13): 3061–3075. doi:10.1002/cncr.32816. PMID 32249416. S2CID 214809009.
- ↑ Jayedi A, Emadi A, Khan TA, Abdolshahi A, Shab-Bidar S (2020). "Dietary Fiber and Survival in Women with Breast Cancer: A Dose-Response Meta-Analysis of Prospective Cohort Studies". Nutrition and Cancer. 73 (9): 1570–1580. doi:10.1080/01635581.2020.1803928. PMID 32795218. S2CID 221132662.
- 1 2 Bradbury KE, Appleby PN, Key TJ (July 2014). "Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC)". The American Journal of Clinical Nutrition. 100 Suppl 1: 394S–398S. doi:10.3945/ajcn.113.071357. PMID 24920034.
- ↑ Spencer JP (May 2008). "Flavonoids: modulators of brain function?". The British Journal of Nutrition. 99 E Suppl 1 (E Suppl 1): ES60–ES77. doi:10.1017/S0007114508965776. PMID 18503736.
- ↑ Romagnolo DF, Selmin OI (2012). "Flavonoids and cancer prevention: a review of the evidence". Journal of Nutrition in Gerontology and Geriatrics. 31 (3): 206–238. doi:10.1080/21551197.2012.702534. PMID 22888839. S2CID 205960210.
- ↑ Jin H, Leng Q, Li C (August 2012). "Dietary flavonoid for preventing colorectal neoplasms". Colorectal Cancer Group. The Cochrane Database of Systematic Reviews. 8 (8): CD009350. doi:10.1002/14651858.CD009350.pub2. PMID 22895989.
- ↑ "Mushrooms in cancer treatment § Mushrooms and cancer". www.cancerresearchuk.org. Cancer Research UK. 30 January 2013. Archived from the original on 2014-07-08.
- ↑ "Soybean". www.cancer.org. American Cancer Society. 17 January 2013. Archived from the original on 2014-08-26.
- ↑ Filippini T, Malavolti M, Borrelli F, Izzo AA, Fairweather-Tait SJ, Horneber M, Vinceti M (March 2020). "Green tea (Camellia sinensis) for the prevention of cancer". The Cochrane Database of Systematic Reviews. 3 (11): CD005004. doi:10.1002/14651858.CD005004.pub3. PMC 7059963. PMID 32118296.
- ↑ "Green Tea". www.cancer.org. American Cancer Society. 4 May 2012. Archived from the original on 2014-08-26.
- ↑ Zhao LG, Li ZY, Feng GS, Ji XW, Tan YT, Li HL, et al. (March 2021). "Tea Drinking and Risk of Cancer Incidence: A Meta-Analysis of Prospective Cohort Studies and Evidence Evaluation". Advances in Nutrition. 12 (2): 402–412. doi:10.1093/advances/nmaa117. PMC 8009746. PMID 33002099.
- ↑ Zhao LG, Li ZY, Feng GS, Ji XW, Tan YT, Li HL, et al. (February 2020). "Coffee drinking and cancer risk: an umbrella review of meta-analyses of observational studies". BMC Cancer. 20 (1): 101. doi:10.1186/s12885-020-6561-9. PMC 7003434. PMID 32024485.
- ↑ Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Krstic G, Wetterslev J, Gluud C (June 2014). "Vitamin D supplementation for prevention of cancer in adults". Metabolic and Endocrine Disorders Group. The Cochrane Database of Systematic Reviews. 6 (6): CD007469. doi:10.1002/14651858.CD007469.pub2. PMID 24953955.
- ↑ Mikol YB, Hoover KL, Creasia D, Poirier LA (December 1983). "Hepatocarcinogenesis in rats fed methyl-deficient, amino acid-defined diets". Carcinogenesis. 4 (12): 1619–1629. doi:10.1093/carcin/4.12.1619. PMID 6317218.
- ↑ Ghoshal AK, Farber E (October 1984). "The induction of liver cancer by dietary deficiency of choline and methionine without added carcinogens". Carcinogenesis. 5 (10): 1367–1370. doi:10.1093/carcin/5.10.1367. PMID 6488458.
- ↑ Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H (November 2001). "A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice". Carcinogenesis. 22 (11): 1871–1875. doi:10.1093/carcin/22.11.1871. PMID 11698351.
- ↑ Henning SM, Swendseid ME, Coulson WF (January 1997). "Male rats fed methyl- and folate-deficient diets with or without niacin develop hepatic carcinomas associated with decreased tissue NAD concentrations and altered poly(ADP-ribose) polymerase activity". The Journal of Nutrition. 127 (1): 30–36. doi:10.1093/jn/127.1.30. PMID 9040540.
- ↑ Caudill MA, Wang JC, Melnyk S, Pogribny IP, Jernigan S, Collins MD, et al. (November 2001). "Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice". The Journal of Nutrition. 131 (11): 2811–2818. doi:10.1093/jn/131.11.2811. PMID 11694601.
- ↑ Poirier LA, Wise CK, Delongchamp RR, Sinha R (June 2001). "Blood determinations of S-adenosylmethionine, S-adenosylhomocysteine, and homocysteine: correlations with diet". Cancer Epidemiology, Biomarkers & Prevention. 10 (6): 649–655. PMID 11401915. Archived from the original on 2012-07-07. Retrieved 2022-12-08.
- ↑ Prinz-Langenohl R, Fohr I, Pietrzik K (June 2001). "Beneficial role for folate in the prevention of colorectal and breast cancer". European Journal of Nutrition. 40 (3): 98–105. doi:10.1007/PL00007387. PMID 11697447. S2CID 39886028.
- ↑ Van den Veyver IB (2002). "Genetic effects of methylation diets". Annual Review of Nutrition. 22: 255–282. doi:10.1146/annurev.nutr.22.010402.102932. PMID 12055346.
- ↑ Cavuoto P, Fenech MF (October 2012). "A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension". Cancer Treatment Reviews. 38 (6): 726–736. doi:10.1016/j.ctrv.2012.01.004. PMID 22342103.
- 1 2 Wang, Zhiyu; Wang, Neng; Liu, Pengxi; Xie, Xiaoming (2016). "AMPK and Cancer". AMP-activated Protein Kinase. Springer International Publishing: 203–226. doi:10.1007/978-3-319-43589-3_9.
- ↑ Carling, David (April 2017). "AMPK signalling in health and disease". Current Opinion in Cell Biology. 45: 31–37. doi:10.1016/j.ceb.2017.01.005.
- ↑ Li, Jin; Zhong, Liping; Wang, Fengzhong; Zhu, Haibo (May 2017). "Dissecting the role of AMP-activated protein kinase in human diseases". Acta Pharmaceutica Sinica B. 7 (3): 249–259. doi:10.1016/j.apsb.2016.12.003.
- ↑ Yung, Mingo M.H.; Ngan, Hextan Y.S.; Chan, David W. (1 April 2016). "Targeting AMPK signaling in combating ovarian cancers: opportunities and challenges". Acta Biochimica et Biophysica Sinica. 48 (4): 301–317. doi:10.1093/abbs/gmv128.
- ↑ Meynet, Ophélie; Ricci, Jean-Ehrland (August 2014). "Caloric restriction and cancer: molecular mechanisms and clinical implications". Trends in Molecular Medicine. 20 (8): 419–427. doi:10.1016/j.molmed.2014.05.001. ISSN 1471-499X.
- 1 2 Fay, Judith R.; Steele, Vernon; Crowell, James A. (1 April 2009). "Energy Homeostasis and Cancer Prevention: The AMP-Activated Protein Kinase". Cancer Prevention Research. 2 (4): 301–309. doi:10.1158/1940-6207.CAPR-08-0166.
- ↑ Skuli, Sarah J.; Alomari, Safwan; Gaitsch, Hallie; Bakayoko, A’ishah; Skuli, Nicolas; Tyler, Betty M. (19 May 2022). "Metformin and Cancer, an Ambiguanidous Relationship". Pharmaceuticals. 15 (5): 626. doi:10.3390/ph15050626.
- ↑ Ingram, Donald K.; Roth, George S. (June 2021). "Glycolytic inhibition: an effective strategy for developing calorie restriction mimetics". GeroScience. 43 (3): 1159–1169. doi:10.1007/s11357-020-00298-7.
- ↑ Guigas, Bruno; Viollet, Benoit (2016). "Targeting AMPK: From Ancient Drugs to New Small-Molecule Activators". AMP-activated Protein Kinase. 107: 327–350. doi:10.1007/978-3-319-43589-3_13.
External links
- "Diet, healthy eating and cancer". info.cancerresearchuk.org. Cancer Research UK. 2013-08-22. Archived from the original on 2012-02-17. Retrieved 2022-12-08.
- "EPIC (European Prospective Investigation into Cancer and Nutrition) Study". epic.iarc.fr. International Agency for Research on Cancer: World Health Organization. Archived from the original on 2023-01-01. Retrieved 2022-12-08.