ELISA
| ELISA | |
|---|---|
| Other names: Enzyme-linked immunosorbent assay[1] | |
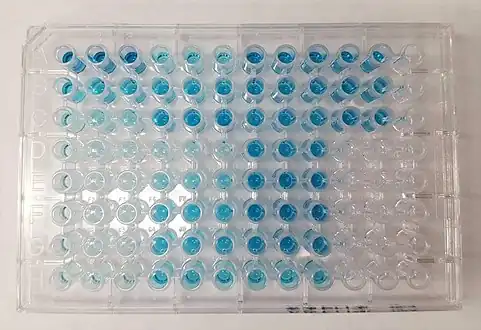 An ELISA being developed with TMB substrate for horseradish peroxidase-linked secondary antibody | |
| Purpose | can help detect[2] [3] HIV SARS West Nile virus Lyme disease |
| MeSH | D004797 |
The ELISA (enzyme-linked immunosorbent assay) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971.[4] The assay uses a solid-phase type of enzyme immunoassay to detect the presence of a ligand (commonly a protein) in a liquid sample using antibodies directed against the protein to be measured. ELISA has been used as a diagnostic tool in medicine, and biotechnology, as well as a quality control check in various industries.[1][5][6]
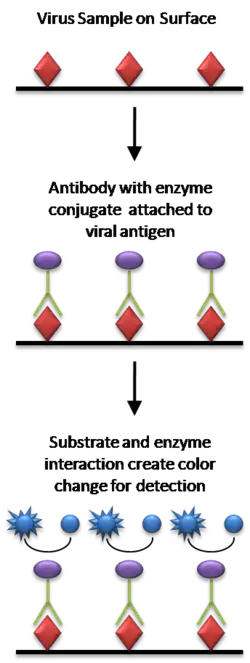
In the most simple form of an ELISA, antigens from the sample to be tested are attached to a surface. Then, a matching antibody is applied over the surface so it can bind the antigen. This antibody is linked to an enzyme and then any unbound antibodies are removed. In the final step, a substance containing the enzyme's substrate is added. If there was binding, the subsequent reaction produces a detectable signal, most commonly a color change.[1][5][7]
Performing an ELISA involves at least one antibody with specificity for a particular antigen. The sample with an unknown amount of antigen is immobilized on a solid support either non-specifically or specifically . After the antigen is immobilized, the detection antibody is added, forming a complex with the antigen. The detection antibody can be covalently linked to an enzyme or can itself be detected by a secondary antibody that is linked to an enzyme through bioconjugation. Between each step, the plate is typically washed with a mild detergent solution to remove any proteins or antibodies that are non-specifically bound. After the final wash step, the plate is developed by adding an enzymatic substrate to produce a visible signal, which indicates the quantity of antigen in the sample.[1][5][8]
ELISA can perform other forms of ligand binding assays instead of strictly "immuno" assays, though the name carried the original "immuno" because of the common use and history of development of this method. The technique essentially requires any ligating reagent that can be immobilized on the solid phase along with a detection reagent that will bind specifically and use an enzyme to generate a signal that can be properly quantified. In between the washes, only the ligand and its specific binding counterparts remain specifically bound or "immunosorbed" by antigen-antibody interactions to the solid phase, while the nonspecific or unbound components are washed away. Unlike other assay formats where the same reaction well can be reused after washing, the ELISA plates have the reaction products immunosorbed on the solid phase, which is part of the plate, and so are not easily reusable.[9][10][1]
Principle

As an analytical biochemistry assay and a "wet lab" technique, ELISA involves detection of an analyte (i.e., the specific substance whose presence is being quantitatively or qualitatively analyzed) in a liquid sample by a method that continues to use liquid reagents during the analysis (i.e., controlled sequence of biochemical reactions that will generate a signal which can be easily quantified and interpreted as a measure of the amount of analyte in the sample) that stays liquid and remains inside a reaction chamber or well needed to keep the reactants contained.[11][12] This is in contrast to "dry lab" techniques that use dry strips. Even if the sample is liquid (e.g., a measured small drop), the final detection step in "dry" analysis involves reading of a dried strip by methods such as reflectometry and does not need a reaction containment chamber to prevent spillover or mixing between samples.[13]
As a heterogenous assay, ELISA separates some component of the analytical reaction mixture by adsorbing certain components onto a solid phase which is physically immobilized. In ELISA, a liquid sample is added onto a stationary solid phase with special binding properties and is followed by multiple liquid reagents that are sequentially added, incubated, and washed, followed by some optical change (e.g., color development by the product of an enzymatic reaction) in the final liquid in the well from which the quantity of the analyte is measured. The quantitative "reading" is usually based on detection of intensity of transmitted light by spectrophotometry, which involves quantitation of transmission of some specific wavelength of light through the liquid (as well as the transparent bottom of the well in the multiple-well plate format).[11][12] The sensitivity of detection depends on amplification of the signal during the analytic reactions. Since enzyme reactions are very well known amplification processes, the signal is generated by enzymes which are linked to the detection reagents in fixed proportions to allow accurate quantification, and thus the name "enzyme-linked".[14]
The analyte is also called the ligand because it will specifically bind or ligate to a detection reagent, thus ELISA falls under the bigger category of ligand binding assays.[11] The ligand-specific binding reagent is "immobilized", i.e., usually coated and dried onto the transparent bottom and sometimes also side wall of a well[15] (the stationary "solid phase"/"solid substrate" here as opposed to solid microparticle/beads that can be washed away), which is usually constructed as a multiple-well plate known as the "ELISA plate". Conventionally, like other forms of immunoassays, the specificity of antigen-antibody type reaction is used because it is easy to raise an antibody specifically against an antigen in bulk as a reagent. Alternatively, if the analyte itself is an antibody, its target antigen can be used as the binding reagent.[16]
Types
There are many ELISA tests for particular molecules that use the matching antibodies. ELISA tests are broken into several types of tests based on how the analytes and antibodies are bonded and used.[17][18] The major types are described here.[19]
Direct ELISA
The steps of direct ELISA are as follows:[20]
- A buffered solution of the antigen to be tested for is added to each well (usually 96-well plates) of a microtiter plate, where it is given time to adhere to the plastic through charge interactions.
- A solution of nonreacting protein, such as bovine serum albumin or casein, is added to each well in order to cover any plastic surface in the well which remains uncoated by the antigen.
- The primary antibody with an attached (conjugated) enzyme is added, which binds specifically to the test antigen coating the well.
- A substrate for this enzyme is then added. Often, this substrate changes color upon reaction with the enzyme.
- The higher the concentration of the primary antibody present in the serum, the stronger the color change. Often, a spectrometer is used to give quantitative values for color strength.
The enzyme acts as an amplifier; even if only few enzyme-linked antibodies remain bound, the enzyme molecules will produce many signal molecules. A major disadvantage of the direct ELISA is that the method of antigen immobilization is not specific; when serum is used as the source of test antigen, all proteins in the sample may stick to the microtiter plate well, so small concentrations of analyte in serum must compete with other serum proteins when binding to the well surface. The sandwich or indirect ELISA provides a solution to this problem, by using a "capture" antibody specific for the test antigen to pull it out of the serum's molecular mixture.[20][21][22][23]
ELISA may be run in a qualitative or quantitative format, qualitative results provide a simple positive or negative result for a sample.[24][25] [26]
The cutoff between positive and negative is determined by the analyst and may be statistical. Two or three times the standard deviation is often used to distinguish positive from negative samples. In quantitative ELISA, the optical density (OD) of the sample is compared to a standard curve, which is typically a serial dilution of a known-concentration solution of the target molecule.[26][21][27]
The use and meaning of the names "indirect ELISA" and "direct ELISA" differs in the literature and on web sites depending on the context of the experiment. When the presence of an antigen is analyzed, the name "direct ELISA" refers to an ELISA in which only a labelled primary antibody is used, and the term "indirect ELISA" refers to an ELISA in which the antigen is bound by the primary antibody which then is detected by a labeled secondary antibody. In the latter case a sandwich ELISA is clearly distinct from an indirect ELISA. When the "primary" antibody is of interest, this antibody is directly detected by the secondary antibody and the term "indirect ELISA" applies to a setting with two antibodies.[28][21][29]
Sandwich ELISA

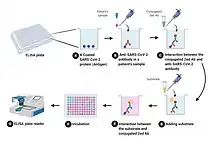
A "sandwich" ELISA is used to detect sample antigen,[30] the steps are:
- A surface is prepared with a known quantity of capture antibody.
- Any nonspecific binding sites on the surface are blocked.
- The antigen-containing sample is applied to the plate, and captured by antibody.
- The plate is washed to remove unbound antigen.
- A specific antibody is added, and binds to antigen (hence the 'sandwich': the antigen is stuck between two antibodies). This primary antibody could be in the serum of a donor, to be tested for reactivity towards the antigen.
- Enzyme-linked secondary antibodies are applied as detection antibodies, which bind specifically to the antibody's Fc region (nonspecific).
- The plate is washed to remove the unbound antibody-enzyme conjugates.
- A chemical is added to be converted by the enzyme into a color, fluorescent, or electrochemical signal.
- The absorbance, fluorescence, or electrochemical signal (e.g., current) of the plate's wells is measured to determine the presence and quantity of the antigen.
The image to the right includes the use of a secondary antibody conjugated to an enzyme, although, in the technical sense, this is not necessary if the primary antibody is conjugated to an enzyme (which would be direct ELISA). However, the use of a secondary-antibody conjugate avoids the expensive process of creating enzyme-linked antibodies for every antigen one might want to detect. By using an enzyme-linked antibody that binds the Fc region of other antibodies, this same enzyme-linked antibody can be used in a variety of situations. Without the first layer of "capture" antibody, any proteins in the sample (including serum proteins) may competitively adsorb to the plate surface, lowering the quantity of antigen immobilized. Use of the purified specific antibody to attach the antigen to the plastic eliminates a need to purify the antigen from complicated mixtures before the measurement, simplifying the assay, and increasing the specificity and the sensitivity of the assay. Therefore, a sandwich ELISA used for research often needs validation, to reduce the risk of false positive results.[31]
Competitive ELISA
A third use of ELISA is through competitive binding. The steps for this ELISA are somewhat different from the first two examples:[32][33][34]
- These bound antibody/antigen complexes are then added to an antigen-coated well.
- The plate is washed, so unbound antibodies are removed.
- The secondary antibody, specific to the primary antibody, is added. This second antibody is coupled to the enzyme.
- A substrate is added, and remaining enzymes elicit a chromogenic or fluorescent signal.
- The reaction is stopped to prevent eventual saturation of the signal.
Some competitive ELISA kits include enzyme-linked antigen rather than enzyme-linked antibody. The labeled antigen competes for primary antibody binding sites with the sample antigen . The less antigen in the sample, the more labeled antigen is retained in the well and the stronger the signal.[35][36][37]
For the detection of HIV antibodies, the wells of microtiter plate are coated with the HIV antigen. Two specific antibodies are used, one conjugated with enzyme and the other present in serum. Cumulative competition occurs between the two antibodies for the same antigen, causing a stronger signal to be seen. If antibodies are present, the antigen-antibody reaction occurs; no antigen is left for the enzyme-labelled specific HIV antibodies. These antibodies remain free upon addition and are washed off during washing. Substrate is added, but there is no enzyme to act on it, so a positive result shows no color change.[38][36][39]
Reverse ELISA
A fourth ELISA test does not use the traditional wells, this test leaves the antigens suspended in the test fluid.[40][41][42]
- Unlabeled antibody is incubated in the presence of its antigen
- A sufficient incubation period is provided to allow the antibodies to bind to the antigens.
- The sample is then passed through the Scavenger container. This can be a test tube or a specifically designed flow through channel. The surface of the Scavenger container or channel has "Scavenger Antigens" bound to it. These can be identical or sufficiently similar to the primary antigens that the free antibodies will bind.
- The Scavenger container must have sufficient surface area and sufficient time to allow the Scavenger Antigens to bind to all the excess Antibodies introduced into the sample.
- The sample, that now contains the tagged and bound antibodies, is passed through a detector. This device can be a flow cytometer or other device that illuminates the tags and registers the response.
This test (which for example can be used for West Nile virus) allows multiple antigens to be tagged and counted at the same time. This allows specific strains of pathogen to be identified by two different color tags; If both tags are present on a cell, then the cell is that specific strain. If only one is present, it is not.This test is done, generally, one test at a time and cannot be done with the microtiter plate.[43]
Enzymatic markers
Lists of enzymatic markers used in ELISA assays, which allow the results of the assay to be measured when complete:
- OPD (o-phenylenediamine dihydrochloride) turns amber to detect HRP (Horseradish Peroxidase), which is often used to as a conjugated protein.[44]
- TMB (3,3',5,5'-tetramethylbenzidine) turns blue when detecting HRP and turns yellow after the addition of sulfuric or phosphoric acid.[44]
- ABTS (2,2'-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt) turns green when detecting HRP.[44]
- PNPP (p-Nitrophenyl Phosphate, Disodium Salt) turns yellow when detecting alkaline phosphatase.[44]
Applications
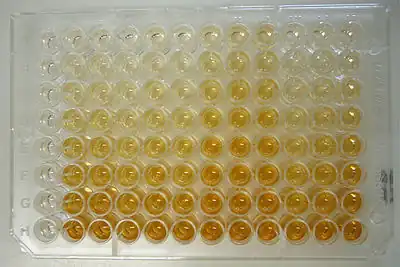
Because the ELISA can be performed to evaluate either the presence of antigen or the presence of antibody in a sample, it is a useful tool for determining serum antibody concentrations (such as with the HIV test[46] or West Nile virus). It has also found applications in the food industry in detecting potential food allergens, such as milk, peanuts, walnuts, almonds, and eggs[47] and as serological blood test for coeliac disease.[48][49] ELISA can also be used in toxicology as a rapid presumptive screen for certain classes of drugs.[50]

The ELISA was the first screening test widely used for HIV because of its high sensitivity.[51]
In an ELISA, a person's serum is diluted 400 times and applied to a plate to which HIV antigens are attached. If antibodies to HIV are present in the serum, they may bind to these HIV antigens. The plate is then washed to remove all other components of the serum. A specially prepared "secondary antibody"—an antibody that binds to other antibodies—is then applied to the plate, followed by another wash. This secondary antibody is chemically linked in advance to an enzyme.Thus, the plate will contain enzyme in proportion to the amount of secondary antibody bound to the plate. A substrate for the enzyme is applied, and catalysis by the enzyme leads to a change in color or fluorescence. ELISA results are reported as a number. A cut-off point may be determined by comparing it with a known standard. If an ELISA test is used for drug screening at workplace, a cut-off concentration, 50ng/ml, for example, is established, and a sample containing the standard concentration of analyte will be prepared. Unknowns that generate a stronger signal than the known sample are positive. Those that generate weaker signal are negative.[52][53][54][55]
There are ELISA tests to detect various kind of diseases, such as dengue, malaria, Chagas disease,[56] Johne's disease, and others.[57] ELISA tests also are extensively employed for in vitro diagnostics in medical laboratories.The other uses of ELISA include detection of SARS-CoV-2 antibodies in blood samples[58][59]
History
Before the development of the ELISA, the only option for conducting an immunoassay was radioimmunoassay, a technique using radioactively labeled antigens or antibodies. In radioimmunoassay, the radioactivity provides the signal, which indicates whether a specific antigen or antibody is present in the sample. Radioimmunoassay was first described in a scientific paper by Rosalyn Sussman Yalow and Solomon Berson published in 1960.[60]
As radioactivity poses a potential health threat, a safer alternative was sought. A suitable alternative to radioimmunoassay would substitute a nonradioactive signal in place of the radioactive signal. When enzymes (such as horseradish peroxidase) react with appropriate substrates (such as ABTS or TMB), a change in color occurs, which is used as a signal. However, the signal has to be associated with the presence of antibody or antigen, which is why the enzyme has to be linked to an appropriate antibody. This linking process was independently developed by Stratis Avrameas and G. B. Pierce.[61] Since it is necessary to remove any unbound antibody or antigen by washing, the antibody or antigen has to be fixed to the surface of the container; i.e., the immunosorbent must be prepared. A technique to accomplish this was published by Wide and Jerker Porath in 1966.[62]
In 1971, Peter Perlmann and Eva Engvall at Stockholm University in Sweden, and Anton Schuurs and Bauke van Weemen in the Netherlands independently published papers that synthesized this knowledge into methods to perform EIA/ELISA.[63][64]
Traditional ELISA typically involves chromogenic reporters and substrates that produce some kind of observable color change to indicate the presence of antigen or analyte. Newer ELISA-like techniques use fluorogenic, electrochemiluminescent, and quantitaoppositiontive PCR reporters to create quantifiable signals. These new reporters can have various advantages, including higher sensitivities and multiplexing.[65][66]
In 2012, an ultrasensitive, enzyme-based ELISA test using nanoparticles as a chromogenic reporter was able to give a naked-eye colour signal, from the detection of mere attograms of analyte. A blue color appears for positive results and red color for negative. Note that this detection only can confirm the presence or the absence of analyte, not the actual concentration.[67]
See also
- Agglutination-PCR
- Immunoscreening
- Lateral flow test
- Magnetic immunoassay
- Microtitre plate
- Nephelometry
- Plaque reduction neutralization test
- Plate reader
- Secretion assay
References
- 1 2 3 4 5 Alhajj, Mandy; Zubair, Muhammad; Farhana, Aisha (2023). "Enzyme Linked Immunosorbent Assay". StatPearls. StatPearls Publishing. PMID 32310382. Archived from the original on 25 September 2021. Retrieved 16 August 2023.
- ↑ "ELISA" (PDF). Bio-Rad. Archived (PDF) from the original on 1 August 2023. Retrieved 15 August 2023.
- ↑ Wang, Chao; Liu, Mei; Wang, Zhifei; Li, Song; Deng, Yan; He, Nongyue (2021). "Point-of-care diagnostics for infectious diseases: From methods to devices". Nano Today. 37: 101092. doi:10.1016/j.nantod.2021.101092. ISSN 1748-0132. Archived from the original on 1 September 2023. Retrieved 31 August 2023.
- ↑ Engvall, E (1972-11-22). "Enzyme-linked immunosorbent assay, Elisa". The Journal of Immunology. 109 (1): 129–135. doi:10.4049/jimmunol.109.1.129. ISSN 0022-1767. PMID 4113792. Archived from the original on 2020-08-05. Retrieved 2023-08-12.
- 1 2 3 Tabatabaei, Mahdis Sadat; Ahmed, Marya (2022). "Enzyme-Linked Immunosorbent Assay (ELISA)". Cancer Cell Biology. Methods in Molecular Biology (Clifton, N.J.). Vol. 2508. pp. 115–134. doi:10.1007/978-1-0716-2376-3_10. ISBN 978-1-0716-2375-6. ISSN 1940-6029. PMID 35737237. Archived from the original on 30 March 2023. Retrieved 18 August 2023.
- ↑ Aydin, Suleyman (October 2015). "A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA". Peptides. 72: 4–15. doi:10.1016/j.peptides.2015.04.012. ISSN 1873-5169. PMID 25908411. S2CID 36486495. Archived from the original on 23 August 2023. Retrieved 22 August 2023.
- ↑ "Step-by-step guide to ELISA | Mabtech". www.mabtech.com. Archived from the original on 5 June 2023. Retrieved 27 August 2023.
- ↑ Clark, Michael F.; Lister, Richard M.; Bar-Joseph, Moshe (1 January 1986). "ELISA techniques". Methods in Enzymology. Academic Press. 118: 742–766. doi:10.1016/0076-6879(86)18114-6. ISBN 9780121820183. Archived from the original on 13 March 2023. Retrieved 23 August 2023.
- ↑ O'Hara, Denise M.; Theobald, Valerie; Egan, Adrienne Clements; Usansky, Joel; Krishna, Murli; TerWee, Julie; Maia, Mauricio; Spriggs, Frank P.; Kenney, John; Safavi, Afshin; Keefe, Jeannine (June 2012). "Ligand binding assays in the 21st century laboratory: recommendations for characterization and supply of critical reagents". The AAPS Journal. 14 (2): 316–328. doi:10.1208/s12248-012-9334-9. ISSN 1550-7416. PMC 3326169. PMID 22415613.
- ↑ Charles A Janeway, Jr; Travers, Paul; Walport, Mark; Shlomchik, Mark J. (2001). "The interaction of the antibody molecule with specific antigen". Immunobiology: The Immune System in Health and Disease. 5th edition. Garland Science. Archived from the original on 17 October 2022. Retrieved 28 August 2023.
- 1 2 3 Msagati, T.A. (2017). Food Forensics and Toxicology. John Wiley & Sons. p. PT229. ISBN 9781119101383. Archived from the original on 2023-08-18. Retrieved 2023-08-12.
- 1 2 Crowther, J.R. (1995). "Chapter 2: Basic Principles of ELISA". ELISA: Theory and Practice. Methods in Molecular Biology. Vol. 42. Humana Press. pp. 35–62. doi:10.1385/0-89603-279-5:1. ISBN 0896032795. PMID 7655571. Archived from the original on 2023-08-18. Retrieved 2023-08-12.
- ↑ Sonntag, O. (1993). "Chapter 1: Introduction to dry chemistry". In van der Vliet, P.C. (ed.). Dry Chemistry: Analysis with carrier-bound reagents. Laboratory Techniques in Biochemistry and Molecular Biology. Vol. 25. pp. 1–6. ISBN 9780080887364. Archived from the original on 2023-08-18. Retrieved 2023-08-12.
- ↑ Hsieh, Y.-H.P.; Rao, Q. (2017). Nielsen, S.S. (ed.). Food Analysis. Springer. pp. 491–98. ISBN 9783319457765. Archived from the original on 2023-08-18. Retrieved 2023-08-12.
- ↑ Schasfoort, R.B.M. (2017). Handbook of Surface Plasmon Resonance (2nd ed.). Royal Society of Chemistry. p. 296. ISBN 9781782627302.
- ↑ Elgert, K.D. (2009). Immunology: Understanding the Immune System. John Wiley & Sons. pp. 149–50. ISBN 9780470081570.
- ↑ R., Crowther, J. (1995). ELISA : theory and practice. Methods in Molecular Biology. Vol. 42. Totowa, N.J.: Humana Press. pp. 1–218. doi:10.1385/0-89603-279-5:1. ISBN 978-0896032798. OCLC 32130600. PMID 7655571.
- ↑ ROBERT., HNASKO (2016). ELISA (SOFTCOVER reprint OF ed.). [Place of publication not identified]: HUMANA. ISBN 978-1493953851. OCLC 960834982.
- ↑ "What is an ELISA?". R&D Systems. Archived from the original on 31 January 2020. Retrieved 31 January 2020.
- 1 2 Spence, Zachary (2018-10-18). "Biochemistry 8th ed - Jeremy M. Berg": 83.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: url-status (link) - 1 2 3 Lin, Alice V. (2015). "Direct ELISA". Methods in Molecular Biology (Clifton, N.J.). 1318: 61–67. doi:10.1007/978-1-4939-2742-5_6. ISSN 1940-6029. Archived from the original on 31 January 2022. Retrieved 26 August 2023.
- ↑ "Bio-Rad antibodies (page 7)" (PDF). Bio-Rad. Archived (PDF) from the original on 3 August 2022. Retrieved 4 September 2023.
- ↑ Stanker, Larry H.; Hnasko, Robert M. (2015). "A Double-Sandwich ELISA for Identification of Monoclonal Antibodies Suitable for Sandwich Immunoassays". ELISA: Methods and Protocols. Springer. pp. 69–78. ISBN 978-1-4939-2742-5. Archived from the original on 2018-06-04. Retrieved 2023-09-05.
- ↑ Sakamoto, Seiichi; Putalun, Waraporn; Vimolmangkang, Sornkanok; Phoolcharoen, Waranyoo; Shoyama, Yukihiro; Tanaka, Hiroyuki; Morimoto, Satoshi (January 2018). "Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites". Journal of Natural Medicines. 72 (1): 32–42. doi:10.1007/s11418-017-1144-z. ISSN 1861-0293. PMC 5775980. PMID 29164507.
- ↑ "ELISA analysis | Abcam". www.abcam.com. Archived from the original on 23 August 2023. Retrieved 20 August 2023.
- 1 2 Hayrapetyan, Hovhannes; Tran, Thao; Tellez-Corrales, Eglis; Madiraju, Charitha (2023). "Enzyme-Linked Immunosorbent Assay: Types and Applications". ELISA. Methods in Molecular Biology (Clifton, N.J.). Vol. 2612. pp. 1–17. doi:10.1007/978-1-0716-2903-1_1. ISBN 978-1-0716-2902-4. ISSN 1940-6029. PMID 36795355. Archived from the original on 11 August 2023. Retrieved 23 August 2023.
- ↑ Arora, Sumitra (8 April 2019). Pesticide Risk Assessment. CABI. p. 112. ISBN 978-1-78064-633-6. Archived from the original on 7 September 2023. Retrieved 4 September 2023.ELISA may be run in a qualitative or quantitative mode. The qualitative results provide positive or negative results (yes or no) in sample, while in quantitative ELISA, the optical density (OD) of the sample is compared to a standard ...
- ↑ Kohl, Thomas O.; Ascoli, Carl A. (1 May 2017). "Direct and Indirect Cell-Based Enzyme-Linked Immunosorbent Assay". Cold Spring Harbor Protocols. 2017 (5): pdb.prot093732. doi:10.1101/pdb.prot093732. ISSN 1559-6095. PMID 28461659. Archived from the original on 17 June 2022. Retrieved 19 August 2023.
- ↑ "ELISA Assays: Indirect, Sandwich, and Competitive | Immunology | JoVE". www.jove.com. Archived from the original on 1 September 2023. Retrieved 29 August 2023.
- ↑ Schmidt, SD; Mazzella, MJ; Nixon, RA; Mathews, PM (2012). Aβ measurement by enzyme-linked immunosorbent assay. Methods in Molecular Biology. Vol. 849. pp. 507–27. doi:10.1007/978-1-61779-551-0_34. ISBN 978-1-61779-550-3. PMID 22528112.
- ↑ Kragstrup, Tue W; Vorup-Jensen, Thomas; Deleuran, Bent; Hvid, Malene (2013). "A simple set of validation steps identifies and removes false results in a sandwich enzyme-linked immunosorbent assay caused by anti-animal IgG antibodies in plasma from arthritis patients". SpringerPlus. 2 (1): 263. doi:10.1186/2193-1801-2-263. PMC 3695686. PMID 23875127.
- ↑ Branch, Angie (22 July 2021). "What is a Competitive ELISA?". Echelon Biosciences. Archived from the original on 3 June 2023. Retrieved 16 August 2023.
- ↑ Shah, Karishma; Maghsoudlou, Panagiotis (2 July 2016). "Enzyme-linked immunosorbent assay (ELISA): the basics". British Journal of Hospital Medicine. 77 (7): C98–C101. doi:10.12968/hmed.2016.77.7.c98. ISSN 1750-8460.
{{cite journal}}: CS1 maint: url-status (link) - ↑ "Competitive ELISA Protocol". www.elisa-antibody.com. Archived from the original on 26 June 2023. Retrieved 2 September 2023.
- ↑ Naar, J; Weidner, A; Baden, DG (2004). "Competitive ELISA: An Accurate, Quick and Effective Tool to Monitor Brevetoxins in Environmental and Biological Sample". Harmful Algae 2002 : Proceedings of the XTH International Conference on Harmful Algae, St. Pete Beach, Florida, USA, October 21-25, 2002 / Edited by Karen A. Steidinger [And Others]. International Conference on Harmful Algae (10Th : 200. 10: 291–293. PMC 4591924. PMID 26436142.
- 1 2 "Types of ELISA". Bio-Rad. Archived from the original on 16 March 2023. Retrieved 22 August 2023.
- ↑ "Overview of ELISA - US". www.thermofisher.com. Archived from the original on 31 May 2023. Retrieved 24 August 2023.
- ↑ "Enzyme-Linked Immunosorbent Assay (ELISA) | NIH". clinicalinfo.hiv.gov. Archived from the original on 23 August 2023. Retrieved 21 August 2023.
- ↑ Gan, Stephanie D.; Patel, Kruti R. (September 2013). "Enzyme Immunoassay and Enzyme-Linked Immunosorbent Assay". Journal of Investigative Dermatology. 133 (9): 1–3. doi:10.1038/jid.2013.287. Archived from the original on 30 January 2023. Retrieved 3 September 2023.
- ↑ US 7767404, Charbonnet, Derrick, "Apparatus and method for single-step immunosorbent assay for single and multiple analytes", issued August 3, 2010
- ↑ US 8735142, Charbonnet, Derrick & Norman Scott Evans, "Systems and methods for immunosorbent assays for single and multiple analytes", issued May 27, 2014
- ↑ Mahmoudi Gomari, Mohammad; Saraygord-Afshari, Neda; Farsimadan, Marziye; Rostami, Neda; Aghamiri, Shahin; Farajollahi, Mohammad M. (December 2020). "Opportunities and challenges of the tag-assisted protein purification techniques: Applications in the pharmaceutical industry". Biotechnology Advances. 45: 107653. doi:10.1016/j.biotechadv.2020.107653. ISSN 0734-9750. PMID 33157154. S2CID 226276355. Archived from the original on 2023-03-11. Retrieved 2023-08-12.
- ↑ Ludolfs, D.; Niedrig, M.; Paweska, J. T.; Schmitz, H. (1 July 2007). "Reverse ELISA for the detection of anti West Nile virus IgG antibodies in humans". European Journal of Clinical Microbiology & Infectious Diseases. 26 (7): 467–473. doi:10.1007/s10096-007-0309-1. PMID 17554572. S2CID 23309736. Archived from the original on 23 August 2023. Retrieved 20 August 2023.
- 1 2 3 4 "Enzyme Substrates for ELISA". Thermo Fisher Scientific - US. Archived from the original on 2018-06-12. Retrieved 2018-06-06.
- ↑ Dhamad, Ahmed E.; Rhida, Muna A. Abdal (7 October 2020). "COVID-19: molecular and serological detection methods". PeerJ. 8: e10180. doi:10.7717/peerj.10180. ISSN 2167-8359. PMC 7547594. PMID 33083156.
- ↑ MedlinePlus Encyclopedia: ELISA/Western blot tests for HIV
- ↑ "Food Allergen Partnership" (Press release). FDA. January 2001. Archived from the original on August 25, 2017. Retrieved August 20, 2015.
- ↑ Sblattero, D.; Berti, I.; Trevisiol, C.; Marzari, R.; Tommasini, A.; Bradbury, A.; Fasano, A.; Ventura, A.; Not, T. (2000). "Human recombinant tissue transglutaminase ELISA: an innovative diagnostic assay for celiac disease". The American Journal of Gastroenterology. 95 (5): 1253–7. doi:10.1111/j.1572-0241.2000.02018.x. PMID 10811336. S2CID 11018740.
- ↑ Porcelli, Brunetta; Ferretti, Fabio; Vindigni, Carla; Terzuoli, Lucia (2014). "Assessment of a Test for the Screening and Diagnosis of Celiac Disease". Journal of Clinical Laboratory Analysis. 30 (1): 65–70. doi:10.1002/jcla.21816. PMC 6807240. PMID 25385391.
- ↑ Abbott, Dustin L.; Limoges, Jennifer F.; Virkler, Kelly J.; Tracy, Seth J.; Sarris, Gregory G. (21 April 2022). "ELISA Screens for Fentanyl in Urine Are Susceptible to False-Positives in High Concentration Methamphetamine Samples". Journal of Analytical Toxicology. 46 (4): 457–459. doi:10.1093/jat/bkab033. ISSN 1945-2403. PMID 33835171. Archived from the original on 7 July 2022. Retrieved 18 August 2023.
- ↑ Alfie, LG; Longueira, YS; Pippo, M; Cruces, L; Quiroga, MF; Turk, G; Laufer, N (1 May 2023). "Increased risk of false-positive HIV ELISA results after COVID-19". AIDS (London, England). 37 (6): 947–950. doi:10.1097/QAD.0000000000003507. PMC 10089928. PMID 36779499.
{{cite journal}}: Check|pmc=value (help) - ↑ Hosseini, Samira; Vázquez-Villegas, Patricia; Rito-Palomares, Marco; Martinez-Chapa, Sergio O. (30 December 2017). Enzyme-linked Immunosorbent Assay (ELISA): From A to Z. Springer. p. 21. ISBN 978-981-10-6766-2. Archived from the original on 23 August 2023. Retrieved 21 August 2023.
- ↑ Chang, Le; Zhao, Junpeng; Guo, Fei; Ji, Huimin; Zhang, Lu; Jiang, Xinyi; Wang, Lunan (2020). "Comparative Evaluation and Measure of Accuracy of ELISAs, CLIAs, and ECLIAs for the Detection of HIV Infection among Blood Donors in China". The Canadian Journal of Infectious Diseases & Medical Microbiology = Journal Canadien des Maladies Infectieuses et de la Microbiologie Medicale. 2020: 2164685. doi:10.1155/2020/2164685. ISSN 1712-9532. PMC 7443234. PMID 32855748.
- ↑ Agius, Ronald; Nadulski, Thomas (June 2014). "Utility of ELISA screening for the monitoring of abstinence from illegal and legal drugs in hair and urine". Drug Testing and Analysis. 6 (Suppl 1): 101–109. doi:10.1002/dta.1644. ISSN 1942-7611. PMID 24817055. S2CID 24548028. Archived from the original on 31 March 2023. Retrieved 24 August 2023.
- ↑ "ELISA for Virus Test". www.elisa-antibody.com. Archived from the original on 5 June 2023. Retrieved 3 September 2023.
- ↑ Del-Rei, Rodrigo Pimenta; Leony, Leonardo Maia; Celedon, Paola Alejandra Fiorani; Zanchin, Nilson Ivo Tonin; Reis, Mitermayer Galvão dos; Gomes, Yara de Miranda; Schijman, Alejandro Gabriel; Longhi, Silvia Andrea; Santos, Fred Luciano Neves (2019-04-18). "Detection of anti-Trypanosoma cruzi antibodies by chimeric antigens in chronic Chagas disease-individuals from endemic South American countries". PLOS ONE. 14 (4): e0215623. Bibcode:2019PLoSO..1415623D. doi:10.1371/journal.pone.0215623. ISSN 1932-6203. PMC 6472793. PMID 30998741.
- ↑ Griffin, J. F. T.; Spittle, E.; Rodgers, C. R.; Liggett, S.; Cooper, M.; Bakker, D.; Bannantine, J. P. (2005). "Immunoglobulin G1 Enzyme-Linked Immunosorbent Assay for Diagnosis of Johne's Disease in Red Deer (Cervus elaphus)". Clinical and Vaccine Immunology. 12 (12): 1401–9. doi:10.1128/CDLI.12.12.1401-1409.2005. PMC 1317074. PMID 16339063.
- ↑ Dhamad, AE; Abdal Rhida, MA (2020). "COVID-19: molecular and serological detection methods". PeerJ. 8: e10180. doi:10.7717/peerj.10180. PMC 7547594. PMID 33083156.
- ↑ Portilho, AI; Gimenes Lima, G; De Gaspari, E (9 March 2022). "Enzyme-Linked Immunosorbent Assay: An Adaptable Methodology to Study SARS-CoV-2 Humoral and Cellular Immune Responses". Journal of Clinical Medicine. 11 (6): 1503. doi:10.3390/jcm11061503. PMC 8948777. PMID 35329828.
- ↑ Yalow, Rosalyn S.; Berson, Solomon A. (1960). "Immunoassay of endogenous plasma insulin in man". The Journal of Clinical Investigation. 39 (7): 1157–75. doi:10.1172/JCI104130. PMC 441860. PMID 13846364.
- ↑ Lequin, R. M. (2005). "Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA)". Clinical Chemistry. 51 (12): 2415–8. doi:10.1373/clinchem.2005.051532. PMID 16179424.
- ↑ Wide, Leif; Porath, Jerker (1966). "Radioimmunoassay of proteins with the use of Sephadex-coupled antibodies". Biochimica et Biophysica Acta (BBA) - General Subjects. 130 (1): 257–60. doi:10.1016/0304-4165(66)90032-8.
- ↑ Engvall, Eva; Perlmann, Peter (1971). "Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G". Immunochemistry. 8 (9): 871–4. doi:10.1016/0019-2791(71)90454-X. PMID 5135623.
- ↑ Van Weemen, B.K.; Schuurs, A.H.W.M. (1971). "Immunoassay using antigen—enzyme conjugates". FEBS Letters. 15 (3): 232–236. doi:10.1016/0014-5793(71)80319-8. PMID 11945853. S2CID 37147723.
- ↑ Leng, S. X.; McElhaney, J. E.; Walston, J. D.; Xie, D.; Fedarko, N. S.; Kuchel, G. A. (2008). "ELISA and Multiplex Technologies for Cytokine Measurement in Inflammation and Aging Research". The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 63 (8): 879–84. doi:10.1093/gerona/63.8.879. PMC 2562869. PMID 18772478.
- ↑ Adler, Michael; Schulz, Sven; Spengler, Mark (2009). "Cytokine Quantification in Drug Development: A comparison of sensitive immunoassay platforms". Chimera Biotech. Archived from the original on 2015-12-22. Retrieved 2023-08-17.
- ↑ de la Rica, Roberto; Stevens, Molly M. (2012). "Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye". Nature Nanotechnology. 7 (12): 821–4. Bibcode:2012NatNa...7..821D. doi:10.1038/nnano.2012.186. hdl:10044/1/21938. PMID 23103935.
External links
- ELISA at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Introduction to ELISA Activity" Archived 2012-03-13 at the Wayback Machine—beginner walk-through of ELISA used for detecting HIV, including animations at the University of Arizona
- "ELISA Assay Principle" Archived 2023-07-20 at the Wayback Machine - An overview of the principle of an ELISA and tools for detecting analyties at Assay Genie Archived 2023-08-10 at the Wayback Machine