Thrombin generation assay
A thrombin generation assay (TGA) or thrombin generation test (TGT) is a global coagulation assay (GCA) and type of coagulation test which can be used to assess coagulation and thrombotic risk.[1][2][3] It is based on the potential of a plasma to generate thrombin over time, following activation of coagulation via addition of phospholipids, tissue factor, and calcium.[4] The results of the TGA can be output as a thrombogram or thrombin generation curve using computer software with calculation of thrombogram parameters.[5][1]
TGAs can be performed with methods like the semi-automated calibrated automated thrombogram (CAT) (2003) or the fully-automated ST Genesia system (2018).[6][1][7] TGAs were first used as manual assays in the 1950s and have since become increasingly automated.[1]
Parameters
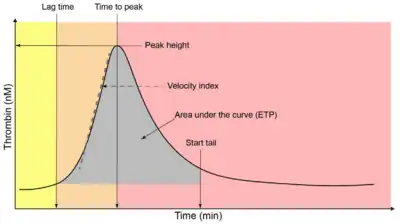
Thrombogram parameters for the TGA include:[1]
- Lag time (minutes; time until thrombin first generated/thrombin concentration first increased)
- Time to peak or ttPeak (minutes; time to maximum concentration of thrombin generated)
- Start tail (minutes; time at which thrombin generation ends and all generated thrombin has been inhibited)
- Peak height or peak thrombin (molar concentration (e.g., nM) of thrombin; peak or maximum concentration of thrombin generated)
- Velocity index (slope of thrombin generation between lag time/first thrombin generation and time to peak; corresponds to first derivative of this part of curve)
- Endogenous thrombin potential (ETP; area under the curve of the thrombin generation curve)
ETP-based APC resistance test

The addition of activated protein C (APC) to a TGA results in an inhibition of thrombin generation as measured by reduction of the endogenous thrombin potential (ETP; area under the thrombin generation curve).[4] This can be used to assess APC resistance and is termed the ETP-based APC resistance test.[4] Results may be expressed as normalized APC sensitivity ratio (nAPCsr), which corresponds to the ratio of the ETP measured in the presence and absence of APC divided by the same ratio in reference plasma.[4] The ratio that is obtained ranges from 0 to 10.[4] In contrast to activated partial thromboplastin time (aPTT)-based APC resistance test (which provides an APCsr value), the higher the nAPCsr value, the greater the APC resistance of the person.[4]
The ETP-based APC resistance test is highly sensitive to procoagulatory changes induced by estrogens (e.g., estrogen-containing birth control pills and estrogen-based hormone therapy) and is much more sensitive to these changes than is the aPTT-based test.[8][9][4][10][6][11]
The ETP-based APC resistance test was developed in 1997, following the development of the aPTT-based test in 1993.[4]
References
- 1 2 3 4 5 6 7 Depasse F, Binder NB, Mueller J, Wissel T, Schwers S, Germer M, Hermes B, Turecek PL (December 2021). "Thrombin generation assays are versatile tools in blood coagulation analysis: A review of technical features, and applications from research to laboratory routine". J Thromb Haemost. 19 (12): 2907–2917. doi:10.1111/jth.15529. PMID 34525255.
- ↑ Binder NB, Depasse F, Mueller J, Wissel T, Schwers S, Germer M, Hermes B, Turecek PL (December 2021). "Clinical use of thrombin generation assays". J Thromb Haemost. 19 (12): 2918–2929. doi:10.1111/jth.15538. PMID 34592058.
- ↑ Duarte RC, Ferreira CN, Rios DR, Reis HJ, Carvalho MD (2017). "Thrombin generation assays for global evaluation of the hemostatic system: perspectives and limitations". Rev Bras Hematol Hemoter. 39 (3): 259–265. doi:10.1016/j.bjhh.2017.03.009. PMC 5568585. PMID 28830606.
- 1 2 3 4 5 6 7 8 9 10 Morimont L, Haguet H, Dogné JM, Gaspard U, Douxfils J (2021). "Combined Oral Contraceptives and Venous Thromboembolism: Review and Perspective to Mitigate the Risk". Front Endocrinol (Lausanne). 12: 769187. doi:10.3389/fendo.2021.769187. PMC 8697849. PMID 34956081.
- ↑ Tripodi A (May 2016). "Thrombin Generation Assay and Its Application in the Clinical Laboratory". Clin Chem. 62 (5): 699–707. doi:10.1373/clinchem.2015.248625. PMID 26955824.
- 1 2 Reda S, Morimont L, Douxfils J, Rühl H (August 2020). "Can We Measure the Individual Prothrombotic or Prohemorrhagic Tendency by Global Coagulation Tests?". Hamostaseologie. 40 (3): 364–378. doi:10.1055/a-1153-5824. PMID 32726831.
- ↑ Kintigh J, Monagle P, Ignjatovic V (January 2018). "A review of commercially available thrombin generation assays". Res Pract Thromb Haemost. 2 (1): 42–48. doi:10.1002/rth2.12048. PMC 6055498. PMID 30046705.
- ↑ Tchaikovski SN, Rosing J (July 2010). "Mechanisms of estrogen-induced venous thromboembolism". Thromb Res. 126 (1): 5–11. doi:10.1016/j.thromres.2010.01.045. PMID 20163835.
- ↑ Hemelaar M, van der Mooren MJ, Rad M, Kluft C, Kenemans P (September 2008). "Effects of non-oral postmenopausal hormone therapy on markers of cardiovascular risk: a systematic review". Fertil Steril. 90 (3): 642–72. doi:10.1016/j.fertnstert.2007.07.1298. PMID 17923128.
- ↑ Douxfils J, Morimont L, Bouvy C (November 2020). "Oral Contraceptives and Venous Thromboembolism: Focus on Testing that May Enable Prediction and Assessment of the Risk". Semin Thromb Hemost. 46 (8): 872–886. doi:10.1055/s-0040-1714140. PMID 33080636.
- ↑ Curvers J, Thomassen MC, Nicolaes GA, Van Oerle R, Hamulyak K, Hemker HC, Tans G, Rosing J (April 1999). "Acquired APC resistance and oral contraceptives: differences between two functional tests". Br J Haematol. 105 (1): 88–94. PMID 10233368.