Elsulfavirine
 | |
| Clinical data | |
|---|---|
| Trade names | Elpida |
| Other names | VM 1500; elpivirine |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| Chemical and physical data | |
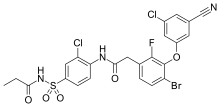
| Formula | C24H17BrCl2FN3O5S |
| Molar mass | 629.28 g·mol−1 |
InChI
| |
Elsulfavirine (trade name Elpida; also known as VM 1500) is drug used to treat HIV infection. It is a non-nucleoside reverse transcriptase inhibitor (NNRTI).[1][2][3] Elsulfavirine is a prodrug which is metabolized to the active antiviral agent deselsulfavirine (also known as VM 1500A).[4] It was developed by the Russian company Viriom.[5]
In June 2017, elsulfavirine was approved for use in Russia as an oral formulation for the treatment of HIV-1 infections in combination with other antiretroviral drugs.[4][6] Currently, elsulfavirine is used in antiretroviral therapy regimens in the Russian Federation, which includes the combination elsulfavirine + lamivudine (or emtricitabine) + tenofovir.[7]
Long-acting injectable formulations of eslulfavarinin and deselsulfavarine are under investigation.[8][9]
In addition, Roche is investigating the use of elsulfavirin for the treatment of COVID-19 and it is currently in Phase II clinical trials for this possible indication.[5]
References
- ↑ Wang Y, De Clercq E, Li G (October 2019). "Current and emerging non-nucleoside reverse transcriptase inhibitors (NNRTIs) for HIV-1 treatment". Expert Opinion on Drug Metabolism & Toxicology. 15 (10): 813–829. doi:10.1080/17425255.2019.1673367. PMID 31556749. S2CID 203439476.
- ↑ Rai MA, Pannek S, Fichtenbaum CJ (June 2018). "Emerging reverse transcriptase inhibitors for HIV-1 infection". Expert Opinion on Emerging Drugs. 23 (2): 149–157. doi:10.1080/14728214.2018.1474202. PMC 6158299. PMID 29737220.
- ↑ Wang Y, De Clercq E, Li G (October 2019). "Current and emerging non-nucleoside reverse transcriptase inhibitors (NNRTIs) for HIV-1 treatment". Expert Opinion on Drug Metabolism & Toxicology. 15 (10): 813–829. doi:10.1080/17425255.2019.1673367. PMID 31556749. S2CID 203439476.
- 1 2 Al-Salama ZT (October 2017). "Elsulfavirine: First Global Approval". Drugs. 77 (16): 1811–1816. doi:10.1007/s40265-017-0820-3. PMID 28940154. S2CID 25316512.
- 1 2 "Elsulfavirine - Viriom". AdisInsight.
- ↑ Bolger CA, Carpenter JE, Dhar TG, Pashine A, Dragovich PS, Cook JH, Gillis EP, Peese KM, Merritt JR. "Chapter 29: To Market, To Market--2017". 2018 Medicinal Chemistry Reviews. 53.
- ↑ "Elpida (elsulfavirine) becomes the preferred first line therapy for treatment of HIV infection in Russia" (Press release). PRN Newswire. February 2, 2021.
- ↑ Akram R, DeSimone Jr J (June 5, 201). "What's in the Pipeline?". Contagion. 4 (3).
- ↑ Bichko V, Rogovoy B, Koryakova A (2017). "Pre-clinical pharmacokinetics of elsufavirine/VM1500A long acting injectable formulations". International Antiviral Society-USA. Poster WEPEA0190.