Emtricitabine
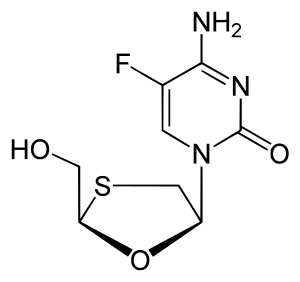 | |
| Names | |
|---|---|
| Pronunciation | /ˌɛmtrəˈsaɪtəbiːn/ |
| Trade names | Emtriva, Coviracil, others |
| Other names | FTC |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604004 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 93% |
| Protein binding | Very low (less than 4%) |
| Metabolism | Liver oxidation and glucuronidation CYP system not involved |
| Elimination half-life | 10 hours |
| Excretion | Kidney (86%) and fecal (14%) |
| Chemical and physical data | |
| Formula | C8H10FN3O3S |
| Molar mass | 247.24 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Emtricitabine (commonly called FTC), with trade name Emtriva, is a medication used to prevent and treat HIV in adults and children.
Emtricitabine is a nucleoside reverse-transcriptase inhibitor (NRTI). It is also marketed as a fixed-dose combination with tenofovir disoproxil as emtricitabine/tenofovir, and with tenofovir alafenamide. A fixed-dose triple combination of emtricitabine, tenofovir and efavirenz in the United States in 2006, Atripla. Emtricitabine makes up one fourth of the Quad pill.
In fixed-dose combinations emtricitabine/tenofovir and emtricitabine/efavirenz/tenofovir are on the World Health Organization's List of Essential Medicines.[2] In 2017, it was the 224th most commonly prescribed medication in the United States, with more than two million prescriptions.[3][4]
Medical uses
HIV
Emtricitabine is indicated in combination with other antiretroviral agents for the prevention and treatment of HIV-1 infection.[5][6]
HBV
Emtricitabine exhibits clinical activity against the hepatitis B virus (HBV), but is not approved by the U.S. Food and Drug Administration (FDA) for the treatment of HBV infection.[5] Among individuals with chronic HBV infection, emtricitabine treatment results in significant histologic, virologic, and biochemical improvement. The safety profile of emtricitabine during treatment is similar to that of a placebo. Emtricitabine, like all other FDA approved drugs, cures neither HIV nor HBV infection. In a study involving individuals with HBV infection, symptoms of infection returned in 23% of emtricitabine-treated individuals who were taken off therapy.[7] In studies involving individuals with chronic HIV infection, viral replication also resumes when study subjects are taken off therapy.[8] As with drugs used to treat HIV infection, drugs used to treat HBV infection may have to be used in combination to prevent the evolution of drug resistant strains. The effectiveness of emtricitabine in combination with other anti-HBV drugs has not been established.
Side effects
In clinical practice, toxicity with emtricitabine is unusual. The most common treatment-related adverse events are diarrhea, headache, nausea, and rash. These symptoms are generally mild to moderate in severity, but they caused 1% of clinical trial patients to give up treatment. Skin discoloration, which is typically reported as hyperpigmentation and usually affects either the palms of the hands or the soles of the feet, is reported in less than 2% of individuals and is almost exclusive to patients of African origin.
Among the more severe side effects patients may experience are a hepatotoxicity or a lactic acidosis.
Mechanism of action
Emtricitabine is an analogue of cytidine. The drug works by inhibiting reverse transcriptase, the enzyme that copies HIV RNA into new viral DNA. By interfering with this process, which is central to the replication of HIV, emtricitabine can help to lower the amount of HIV, or "viral load", in a patient's body and can indirectly increase the number of immune system cells (namely T cells/CD4+ T-cells). Both of these changes are associated with healthier immune systems and decreased likelihood of serious illness.
History
Emtricitabine was discovered by Dr. Dennis C. Liotta, Dr. Raymond F. Schinazi, and Dr. Woo-Baeg Choi of Emory University and licensed to Triangle Pharmaceuticals by Emory in 1996.[9] Triangle Pharmaceuticals was acquired in 2003 by Gilead Sciences, who completed development and now market the product with the brand name Emtriva.
It was approved by the FDA July 2, 2003.[10] It is very similar to lamivudine (3TC) and cross-resistance between the two is near-universal.
Society and culture
Cost
In 2017, it was the 224th most commonly prescribed medication in the United States, with more than two million prescriptions.[3][4]
.svg.png.webp) Emtricitabine costs (US)
Emtricitabine costs (US).svg.png.webp) Emtricitabine prescriptions (US)
Emtricitabine prescriptions (US)
References
- ↑ US 5814639, Liotta DC, Schinazi RF, Choi WB, "Method for the synthesis, compositions and use of 2'-deoxy-5-fluoro-3'-thiacytidine and related compounds", issued 29 September 1998, assigned to Emory University
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 11 April 2020.
- 1 2 "Emtricitabine - Drug Usage Statistics". ClinCalc. Archived from the original on 8 July 2020. Retrieved 11 April 2020.
- 1 2 "Emtriva- emtricitabine capsule Emtriva- emtricitabine solution". DailyMed. 14 December 2018. Archived from the original on 24 July 2020. Retrieved 24 July 2020.
- ↑ "Emtriva EPAR". European Medicines Agency. 17 September 2018. Archived from the original on 24 July 2020. Retrieved 24 July 2020.
- ↑ Lim SG, Ng TM, Kung N, Krastev Z, Volfova M, Husa P, et al. (January 2006). "A double-blind placebo-controlled study of emtricitabine in chronic hepatitis B". Archives of Internal Medicine. 166 (1): 49–56. doi:10.1001/archinte.166.1.49. PMID 16401810.
- ↑ Oxenius A, Price DA, Günthard HF, Dawson SJ, Fagard C, Perrin L, et al. (October 2002). "Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection". Proceedings of the National Academy of Sciences of the United States of America. 99 (21): 13747–52. Bibcode:2002PNAS...9913747O. doi:10.1073/pnas.202372199. PMC 129766. PMID 12370434.
- ↑ Leaf C (September 19, 2005). "The Law of Unintended Consequences". CNN. Archived from the original on November 6, 2020. Retrieved August 3, 2020.
- ↑ Standard & Poor's 500 Guide Archived 2021-07-09 at the Wayback Machine. Standard & Poor's, McGraw-Hill, (2004), p. 83.
External links
| External sites: |
|
|---|---|
| Identifiers: |