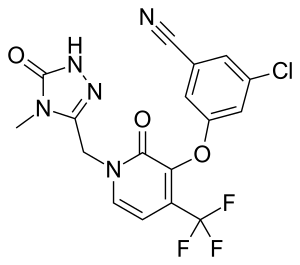
Doravirine
 | |
| Names | |
|---|---|
| Trade names | Pifeltro |
| Other names | MK-1439 |
IUPAC name
| |
| Clinical data | |
| Drug class | Non-nucleoside reverse transcriptase inhibitor (NNRTI)[1] |
| Main uses | HIV/AIDS[1] |
| Side effects | Nausea, dizziness, headache, tiredness, diarrhea, abnormal dreams[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth[1] |
| Typical dose | 100 mg OD[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618048 |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C17H11ClF3N5O3 |
| Molar mass | 425.75 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Doravirine, sold under the brand name Pifeltro, is a medication used to treat HIV/AIDS.[1] It is taken together with other HIV medicines.[1] It is taken by mouth, generally once per day.[1]
Common side effects include nausea, dizziness, headache, tiredness, diarrhea, and abnormal dreams.[1] Other side effects may include immune reconstitution syndrome.[1] Safety in pregnancy is unclear.[4] It is a non-nucleoside reverse transcriptase inhibitor (NNRTI).[1]
Doravirine was approved for medical use in the United States and Europe in 2018.[2] In the United Kingdom a month of medication costs the NHS about £470 as of 2021.[5] This amount in the United States is about 1,500 USD.[6] It is also available as the combination doravirine/lamivudine/tenofovir.[5]
Medical uses
Dosage
It is generally taken at a dose of 100 mg per day.[2]
References
- 1 2 3 4 5 6 7 8 9 10 11 "Pifeltro- doravirine tablet, film coated". DailyMed. 10 October 2019. Archived from the original on 28 October 2020. Retrieved 22 September 2020.
- 1 2 3 4 "Pifeltro EPAR". European Medicines Agency (EMA). Archived from the original on 28 October 2020. Retrieved 1 October 2020.
- ↑ "Pifeltro 100 mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). Archived from the original on 26 September 2020. Retrieved 1 October 2020.
- ↑ "Doravirine (Pifeltro) Use During Pregnancy". Drugs.com. Archived from the original on 3 December 2020. Retrieved 27 December 2021.
- 1 2 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 683. ISBN 978-0857114105.
- ↑ "Pifeltro Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 27 December 2021.
External links
| External sites: |
|
|---|---|
| Identifiers: |