Amdoxovir
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| Chemical and physical data | |
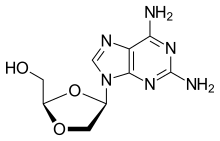
| Formula | C9H12N6O3 |
| Molar mass | 252.234 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Amdoxovir is a pharmaceutical drug that has undergone research for the treatment of HIV/AIDS. It acts as a nucleoside reverse transcriptase inhibitor (NRTI). The drug was discovered by Raymond F. Schinazi (Emory University) and C.K. Chu (University of Georgia) and developed by RFS Pharma.[1]
Amdoxovir was in advanced Phase II clinical trials around 2010.[2] In 2013, a Phase II trial was terminated[3] and another was withdrawn before it started.[4] No further studies appear to have been done.
References
- ↑ "Amdoxovir". AIDSmeds.com. January 13, 2009. Archived from the original on 2008-03-21. Retrieved March 21, 2008.
- ↑ Murphy RL, Kivel NM, Zala C, Ochoa C, Tharnish P, Mathew J, Pascual ML, Schinazi RF (2010). "Antiviral activity and tolerability of amdoxovir with zidovudine in a randomized double-blind placebo-controlled study in HIV-1-infected individuals". Antivir Ther. 15 (2): 185–192. doi:10.3851/IMP1514. PMC 7733239. PMID 20386073.
- ↑ Clinical trial number NCT01737359 for "A Safety and Efficacy Study of Amdoxovir in HIV-1 Treatment-experienced Subjects" at ClinicalTrials.gov
- ↑ Clinical trial number NCT01738555 for "A Safety and Efficacy Study of Amdoxovir in HIV-1 Treatment-experienced Subjects" at ClinicalTrials.gov
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.