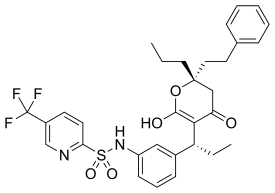
Tipranavir
 | |
| Names | |
|---|---|
| Pronunciation | /tɪpˈrænəvɪər/ tip-RAN-ə-veer |
| Trade names | Aptivus |
| Other names | Tipranavir disodium |
IUPAC name
| |
| Clinical data | |
| Drug class | Protease inhibitor (PI)[1] |
| Main uses | HIV/AIDS |
| Side effects | Diarrhea, nausea, fever, tiredness, intracranial bleeding, liver inflammation, high blood sugar,abnormal lipid levels[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth (soft capsules) |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606009 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 99.9% |
| Metabolism | Liver |
| Elimination half-life | 4.8–6 hours |
| Excretion | Feces (82.3%), urine (4.4%) |
| Chemical and physical data | |
| Formula | C31H33F3N2O5S |
| Molar mass | 602.67 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Tipranavir (TPV), sold under the brand name Aptivus, is a medication used to treat HIV/AIDS in combination with ritonavir among others medications.[1] It is used with similar medications are not effective.[1] It is taken by mouth.[1] It is used in those over the age of two years.[3]
Common side effects include diarrhea, nausea, fever, tiredness, and headache.[1] Other side effects may include intracranial bleeding, liver inflammation, liver failure, high blood sugar, and abnormal lipid levels.[1] It is a protease inhibitor (PI) which blocks HIV protease.[1]
Tipranavir was approved for medical use in the United States and Europe in 2005.[1][3] In the United Kingdom it costs the NHS about £440 a month as of 2021.[4] In the United States this amount costs about 1,800 USD.[5]
Medical uses
Tipranavir should only be taken in combination with ritonavir and other antiretroviral drugs, and is not approved for treatment-naïve patients.[2] Like lopinavir and atazanavir, it is very potent and is effective in salvage therapy for people with drug resistance.
Dosage
It is taken at a dose of 500 mg twice per day.<re name=BNF80/>
Side effects
Aptivus labeling has a black box warning regarding hepatotoxicity and intracranial hemorrhage.[2]
Mechanism of action
Tipranavir has the ability to inhibit the replication of viruses that are resistant to other protease inhibitors and it recommended for patients who are resistant to other treatments. Resistance to tipranavir itself seems to require multiple mutations.[6]
References
- 1 2 3 4 5 6 7 8 9 "Tipranavir Monograph for Professionals". Drugs.com. Archived from the original on 26 August 2021. Retrieved 4 October 2021.
- 1 2 3 "Aptivus- tipranavir capsule, liquid filled Aptivus- tipranavir solution". DailyMed. 26 June 2020. Archived from the original on 22 October 2021. Retrieved 2 December 2020.
- 1 2 "Aptivus". Archived from the original on 14 April 2021. Retrieved 4 October 2021.
- ↑ BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 697. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Aptivus Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 5 August 2016. Retrieved 4 October 2021.
- ↑ Doyon L, Tremblay S, Bourgon L, Wardrop E, Cordingley MG (October 2005). "Selection and characterization of HIV-1 showing reduced susceptibility to the non-peptidic protease inhibitor tipranavir". Antiviral Research. 68 (1): 27–35. doi:10.1016/j.antiviral.2005.07.003. PMID 16122817.
External links
| External sites: |
|
|---|---|
| Identifiers: |