MK-2048
 MK-2048 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.233.568 |
| Chemical and physical data | |
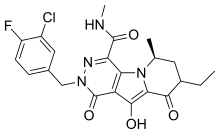
| Formula | C21H21ClFN5O4 |
| Molar mass | 461.88 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
MK-2048 is the Merck & Co. designation for a molecule in its pre-clinical drug discovery that is an integrase inhibitor-class of agent intended to be used against HIV infection.[1] It is a second generation integrase design thought to be superior to the first available integrase inhibitor, raltegravir, in that "MK-2048 has a dissociation half-life of 32 hours on wild-type integrase—more than four times that of raltegravir",[1][2] and its dissociation half-life against the important HIV integrase mutant N155H was on the same order of magnitude as that of raltegravir against wild-type virus, leading the Merck presenter to suggest the possibility of "reduced susceptibility to resistance mutations" for the second generation drug.[1] MK-2048 is being investigated for use as part of a pre-exposure prophylaxis (PrEP) approach to the treatment of HIV infection.[3] At the time of these reports, there was no indication of the time by which "MK-2048, or related compounds, [would] be ready for clinical trials".[1]
References
- 1 2 3 4 Mascolini M (April 17, 2009). "Conference Reports for NATAP: Merck Offers Unique Perspective on Second-Generation Integrase Inhibitor [10th International Workshop on Clinical Pharmacology of HIV Therapy, April 15–17, 2009, Amsterdam]". NATAP.org. Retrieved November 8, 2009.
- ↑ Grobler JA, McKenna PM, Ly S; et al. (April 17, 2009), "Presentation, Abstract O-10: Functionally Irreversible Inhibition of Integration by Slowly Dissociating Strand Transfer Inhibitors. [10th International Workshop on Clinical Pharmacology of HIV Therapy, April 15–17, 2009, Amsterdam]", NATAP.org
{{citation}}: CS1 maint: multiple names: authors list (link) - ↑ Alcorn K (April 28, 2009). "Ralvetgravir Shows Potential for use as PrEP Drug". AIDSmap.com. Archived from the original on January 3, 2010. Retrieved November 8, 2009.