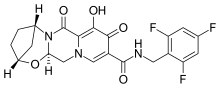
Bictegravir
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C21H18F3N3O5 |
| Molar mass | 449.386 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Bictegravir (INN; BIC, formerly known as GS-9883)[1][2] is a second-generation integrase inhibitor (INSTI) class that was structurally derived from an earlier compound dolutegravir by scientists at Gilead Sciences; in vitro and clinical results were presented by Gilead in the summer of 2016.[3][4] In 2016, bictegravir was in a Phase 3 trial as part of a single tablet regimen in combination with tenofovir alafenamide (TAF) and emtricitabine (FTC) for the treatment of HIV-1 infection[5] and the combination drug bictegravir/emtricitabine/tenofovir alafenamide (Biktarvy) was approved for use in the United States in 2018.[6]
Medical use
Bictegravir is used a in fixed dose combination with tenofovir alafenaminde and emtricitabine for the treatment of HIV-1 infection.[4][7]
Contraindication
Bictegravir is should not be used with dofetilide and rifampin.[8] Use of dofetilide with bictegravir increases the concentration of dofetilide, which can lead to life threatening events.[8] Concomitant use of bictegravir and rifampin causes significant interactions because of an effect rifampin has on bictegravir.[8] Bictagravir is metabolized primarily through the liver (CYP3A4), thus inducer of CYP3A4 should be avoided.[4]
Adverse effects
The most common side effects seen in bictegravir use include diarrhea, nausea, and headache.[4]
References
- ↑ "Recommended INN: List 75" (PDF). WHO Drug Information. 30 (1): 102. 2016.
- ↑ "Bictegravir - Gilead Sciences". Adis Insight. Springer Nature Switzerland AG. Retrieved 22 January 2017.
- ↑ Highleyman L (6 July 2016). "New integrase inhibitor bictegravir looks promising in early studies". NAM aidsmap.
- 1 2 3 4 Zeuli, J; Rizza, S; Bhatia, R; Temesgen, Z (2019-11-01). "Bictegravir, a novel integrase inhibitor for the treatment of HIV infection". Drugs of Today. 55 (11): 669–682. doi:10.1358/dot.2019.55.11.3068796. ISSN 1699-4019. PMID 31840682. S2CID 209385285.
- ↑ "Press Release: Gilead Presents Preliminary Data on Bictegravir, an Investigational Integrase Strand Transfer Inhibitor for the Treatment of HIV | Gilead". Gilead. June 20, 2016.
- ↑ "U.S. Food and Drug Administration Approves Gilead's Biktarvy (Bictegravir, Emtricitabine, Tenofovir Alafenamide) for Treatment of HIV-1 Infection" (Press release). Gilead. February 7, 2018.
- ↑ Wohl, David A.; Yazdanpanah, Yazdan; Baumgarten, Axel; Clarke, Amanda; Thompson, Melanie A.; Brinson, Cynthia; Hagins, Debbie; Ramgopal, Moti N.; Antinori, Andrea; Wei, Xuelian; Acosta, Rima (2019). "Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial". The Lancet. HIV. 6 (6): e355–e363. doi:10.1016/S2352-3018(19)30077-3. ISSN 2352-3018. PMID 31068270. S2CID 148570850.
- 1 2 3 "Biktarvy - FDA Prescribing Highlights" (PDF).
Further reading
- Tsiang M, Jones GS, Goldsmith J, Mulato A, Hansen D, Kan E, et al. (December 2016). "Antiviral Activity of Bictegravir (GS-9883), a Novel Potent HIV-1 Integrase Strand Transfer Inhibitor with an Improved Resistance Profile". Antimicrobial Agents and Chemotherapy. 60 (12): 7086–7097. doi:10.1128/AAC.01474-16. PMC 5118987. PMID 27645238.
External links
- "Bictegravir". Drug Information Portal. U.S. National Library of Medicine.