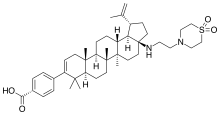
BMS-955176
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| CAS Number | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C42H62N2O4S |
| Molar mass | 691.03 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
BMS-955176 is an experimental second generation HIV maturation inhibitor under development by Bristol-Myers Squibb for use in the treatment of HIV infection. By blocking the maturation of the virus, it prevents viral reproduction in host CD4+ T cells.[1] First generation maturation inhibitors such as bevirimat were ineffective against some naturally occurring changes (polymorphisms) in the Gag protease polyprotein; BMS-955176 has been selected to better tolerate gag polymorphisms.[2][3]
Studies
Results of a phase 2a trial of BMS-955176 was reported at the 2015 Conference on Retroviruses and Opportunistic Infections (CROI).[4] Investigators concluded that the drug was well tolerated and effective against HIV, including strains with gag polymorphisms.[4][5] Phase 2b studies are currently ongoing in early 2016.[6][7] It appears that development of BMS-955176 has been terminated.[8]
References
- ↑ "BMS Maturation Inhibitor Is Potent Against HIV in Early Trial". 25 February 2015. Archived from the original on 23 July 2015. Retrieved 22 July 2015.
- ↑ "CROI2015: New HIV maturation inhibitor BMS-955176 appears more potent than earlier beviramat - Project Inform".
- ↑ Wang D, Lu W, Li F (November 2015). "Pharmacological intervention of HIV-1 maturation". Acta Pharmaceutica Sinica B. 5 (6): 493–9. doi:10.1016/j.apsb.2015.05.004. PMC 4675807. PMID 26713265.
- 1 2 "Antiviral Activity/Safety of a Second-Generation HIV-1 Maturation Inhibitor | CROI Conference". www.croiconference.org. Retrieved 2016-01-01.
- ↑ "HIV maturation inhibitor BMS-955176 looks promising in early study". www.aidsmap.com. Retrieved 2016-01-01.
- ↑ "Dose-finding Study of BMS-955176 to Treat HIV-1 Infected Treatment-naive Adults - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-01-01.
- ↑ Clinical trial number NCT02386098 for "Strategy-confirming Study of BMS-955176 to Treat HIV-1 Infected Treatment-experienced Adults" at ClinicalTrials.gov
- ↑ "GSK Discontinues Development of Maturation Inhibitor BMS-955176".