Loviride
 | |
| Clinical data | |
|---|---|
| Other names | R089439 |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
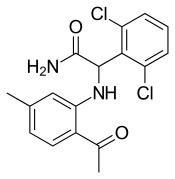
| Formula | C17H16Cl2N2O2 |
| Molar mass | 351.23 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Loviride (also called loveride) was an antiviral drug manufactured by Janssen (now part of Janssen-Cilag) that is active against HIV. Loviride is a non-nucleoside reverse transcriptase inhibitor (NNRTI) that entered phase III clinical trials in the late 1990s but failed to gain marketing approval because of poor potency. It is of clinical significance only in those patients who were enrolled in clinical trials to evaluate loviride (e.g., CAESAR and AVANTI), because in those trials loviride was often given alone and with no companion drug, leading to a high probability of developing reverse transcriptase mutations such as K103N which result in cross-class resistance the NNRTIs efavirenz and nevirapine.
Synthesis
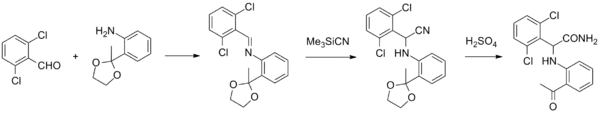
Loviride synthesis: Janssen Pharmaceutica U.S. Patent 5,407,961 (1995).
External links
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.