Mozenavir
 | |
| Clinical data | |
|---|---|
| Trade names | Mozenavir |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
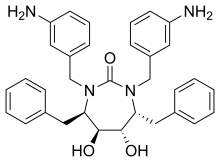
| Formula | C33H36N4O3 |
| Molar mass | 536.7 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Mozenavir (DMP-450) is an antiviral drug which was developed as a treatment for HIV/AIDS. It acts as an HIV protease inhibitor and binds to this target with high affinity,[1][2] however despite promising results in early testing,[3][4] mozenavir was unsuccessful in human clinical trials. Studies continue into related derivatives.[5]
References
- ↑ Nugiel DA, Jacobs K, Kaltenbach RF, Worley T, Patel M, Meyer DT, et al. (May 1996). "Preparation and structure-activity relationship of novel P1/P1'-substituted cyclic urea-based human immunodeficiency virus type-1 protease inhibitors". Journal of Medicinal Chemistry. 39 (11): 2156–69. doi:10.1021/jm960083n. PMID 8667359.
- ↑ Patel M, Bacheler LT, Rayner MM, Cordova BC, Klabe RM, Erickson-Viitanen S, Seitz SP (April 1998). "The synthesis and evaluation of cyclic ureas as HIV protease inhibitors: modifications of the P1/P1' residues". Bioorganic & Medicinal Chemistry Letters. 8 (7): 823–8. doi:10.1016/s0960-894x(98)00119-x. PMID 9871548.
- ↑ De Clercq E (April 2002). "Highlights in the development of new antiviral agents". Mini Reviews in Medicinal Chemistry. 2 (2): 163–75. doi:10.2174/1389557024605474. PMID 12370077.
- ↑ Hensen C, Hermann JC, Nam K, Ma S, Gao J, Höltje HD (December 2004). "A combined QM/MM approach to protein--ligand interactions: polarization effects of the HIV-1 protease on selected high affinity inhibitors". Journal of Medicinal Chemistry. 47 (27): 6673–80. doi:10.1021/jm0497343. PMID 15615516.
- ↑ Li P, Wang S, Wang H, Yan H (2019). "2-Symmetric HIV-1 Protease Inhibitors and Docking Study". Biological & Pharmaceutical Bulletin. 42 (2): 261–267. doi:10.1248/bpb.b18-00705. PMID 30713256.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.