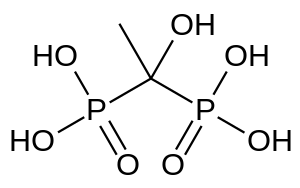
Etidronic acid
 | |
| Names | |
|---|---|
| Trade names | Didronel, others |
| Other names | 1-Hydroxyethylidene-1,1-diphosphonic acid; HEDP |
IUPAC name
| |
| Clinical data | |
| Drug class | Bisphosphonate[1] |
| Main uses | Osteoporosis due to steroids, Paget disease of bone, heterotopic ossification[1] |
| Side effects | Nausea, diarrhea, esophageal problems[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 3% |
| Metabolism | Nil |
| Elimination half-life | 1 to 6 hours |
| Excretion | Kidney and fecal |
| Chemical and physical data | |
| Formula | C2H8O7P2 |
| Molar mass | 206.027 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Etidronic acid, also known as etidronate, is a medication used to treat osteoporosis due to steroids, Paget disease of bone, and heterotopic ossification.[1] It is taken by mouth.[1] Effects may persist for more than 9 months after it is stopped.[1]
Common side effects include nausea and diarrhea.[1] Other side effects may include esophagitis, esophageal perforation, osteonecrosis of the jaw, and potentially esophageal cancer.[1] It is a bisphosphonate.[1] It works by binding to bone and preventing both its growth and its breakdown.[1]
Etidronic acid was patented in 1966 and approved for medical use in 1977.[2] It is available as a generic medication.[3] In the United States it costs about 1.7 USD per 200 mg tablet as of 2021.[3] Other uses include in detergents, water treatment, and cosmetics.[4]
Medical uses
Etidronic acid is a bisphosphonate used to strengthen bone, treat osteoporosis, and treat Paget's disease of bone.
Bisphosphonates primarily reduce osteoclastic activity, which prevents bone resorption, and thus moves the bone resorption/formation equilibrium toward the formation side and hence makes bone stronger on the long run. Etidronate, unlike other bisphosphonates, also prevents bone calcification. For this reason, other bisphosphonates, such as alendronate, are preferred when treating osteoporosis. To prevent bone resorption without affecting too much bone calcification, etidronate must be administered only for a short time once in a while, for example for two weeks every 3 months. When given on a continuous basis, say every day, etidronate will altogether prevent bone calcification. This effect may be useful and etidronate is used this way to to address heterotopic ossification. But in the long run, if used on a continuous basis, it will cause osteomalacia.
Pharmacology
| Bisphosphonate | Relative potency |
|---|---|
| Etidronate | 1 |
| Tiludronate | 10 |
| Pamidronate | 100 |
| Alendronate | 100-500 |
| Ibandronate | 500-1000 |
| Risedronate | 1000 |
| Zoledronate | 5000 |
Synthesis
Etidronic acid can be prepared by reaction of Phosphorus trichloride with acetic acid in a tertiary amine, or by reaction of an acetic acid/acetic anhydride mixture with phosphorous acid.[6]
Other uses
Chemical
HEDP is used as a retardant in concrete, scale and corrosion inhibition in circulating cool water system, oil field and low-pressure boilers in fields such as electric power, chemical industry, metallurgy, fertilizer, etc. In light woven industry, HEDP is used as detergent for metal and nonmetal. In dyeing industry, HEDP is used as peroxide stabilizer and dye-fixing agent; In non-cyanide electroplating, HEDP is used as chelating agent. The dosage of 1–10 mg/L is preferred as scale inhibitor, 10–50 mg/L as corrosion inhibitor, and 1000–2000 mg/L as detergent. Usually, HEDP is also used together with polycarboxylic acid (superplasticizer), in which it acts as reducing agent.
Chelating agent and antioxidant
Etidronic acid is a chelating agent and may be added to bind or, to some extent, counter the effects of substances, such as calcium, iron or other metal ions, which may be discharged as a component of grey wastewater and could conceivably contaminate groundwater supplies. As a phosphonate it has corrosion inhibiting properties on unalloyed steel. Etidronic acid also acts to retard rancidification and oxidation of fatty acids.
HEDP and its salts are added to detergents and other cleaning agents to prevent the effects of hard water. It is also used in peroxide bleaching to prevent degradation of peroxides by transition metals.
Etidronic acid is listed as an ingredient of several cosmetic formulations where it is used for suppressing radical formation, emulsion stabiliser and viscosity control. While etidronic acid has not been limited from inclusion in cosmetics and does have legitimate uses, it is recommended that, as with most cosmetic products (particularly soaps), the product should be thoroughly rinsed from the skin after use.
Etidronic acid is also included among swimming pool chemicals. It is used as a stain inhibitor to prevent metal ions coming out of solution and staining the sides of swimming pools.
References
- 1 2 3 4 5 6 7 8 9 10 "Etidronate Monograph for Professionals". Drugs.com. Archived from the original on 26 January 2021. Retrieved 23 July 2021.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 523. ISBN 9783527607495. Archived from the original on 2021-03-18. Retrieved 2021-03-16.
- 1 2 "Etidronate Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 23 July 2021.
- ↑ Schwab, Manfred (14 October 2011). Encyclopedia of Cancer. Springer Science & Business Media. p. 1338. ISBN 978-3-642-16482-8. Archived from the original on 28 August 2021. Retrieved 23 July 2021.
- ↑ Tripathi KD (2013-09-30). Essentials of medical pharmacology (Seventh ed.). New Delhi. ISBN 9789350259375. OCLC 868299888.
- ↑ "Compound Summary for CID 3305". pubchem.ncbi.nlm.nih.gov. Use and manufacturing. Archived from the original on 28 November 2019. Retrieved 3 February 2015.
External links
| Identifiers: |
|---|