Pamidronic acid
 | |
| Names | |
|---|---|
| Trade names | Aredia, Pamimed, among others |
| Other names | Pamidronate disodium pentahydrate, pamidronate disodium (APD) |
IUPAC name
| |
| Clinical data | |
| Drug class | Bisphosphonate[1] |
| Main uses | High calcium due to cancer, Paget's disease of the bone, bone metastasis that are osteolytic[1] |
| Side effects | Redness and pain at the site of injection, fever, low potassium, low phosphate, bone pain, headache[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intravenous |
| Typical dose | 30 mg to 90mg[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601163 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | n/a |
| Protein binding | 54% |
| Metabolism | Nil |
| Elimination half-life | 28 ± 7 hours |
| Excretion | Kidney |
| Chemical and physical data | |
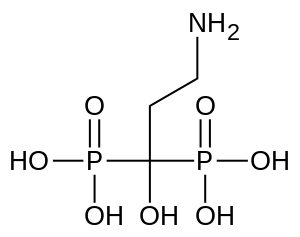
| Formula | C3H11NO7P2 |
| Molar mass | 235.069 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pamidronic acid, also known as pamidronate, is a medication used to treat high calcium due to cancer, Paget's disease of the bone, and bone metastasis that are osteolytic.[1] It is given by gradual injection into a vein.[1]
Common side effects include redness and pain at the site of injection, fever, low potassium, low phosphate, bone pain, and headache.[1] Use during pregnancy may harm the baby.[2] It is a bisphosphonate.[1]
Pamidronic acid was patented in 1971 and approved for medical use in 1987.[3] It is available as a generic medication.[1] In the United States it costs less than 70 USD per dose as of 2021.[4] It is sold under the brand name Aredia among others.[1]
Medical uses
It is used to prevent bone loss, and treat osteoporosis. It is also used to strengthen bone in Paget's disease, to prevent bone loss due to steroid use, and in certain cancers with high propensity to bone, such as multiple myeloma. Due to its ability to sequester calcium in bone, it is also used to treat high calcium levels. It is also used as an experimental treatment of the bone disorder osteogenesis imperfecta. It has been studied in the treatment of complex regional pain syndrome.[5]
Dosage
Intravenous, usually 30 mg to 90 mg.[1] The frequency of the doses depends on the indication.[1] 30 mg, 60 mg, 90 mg and for hospitals, 120 mg vials are available, mixed with mannitol.
Side effects
Common side effects include bone pain, low calcium levels, nausea, and dizziness. Osteonecrosis of the jaw is a rare complication which has been associated with the use of bisphosphonates, including pamidronate.[6]
Pamidronate activates human γδ T cells in vitro and in vivo, which may lead to flu-like symptoms upon administration.
Pharmacology
| Bisphosphonate | Relative potency |
|---|---|
| Etidronate | 1 |
| Tiludronate | 10 |
| Pamidronate | 100 |
| Alendronate | 100-500 |
| Ibandronate | 500-1000 |
| Risedronate | 1000 |
| Zoledronate | 5000 |
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "Pamidronate Monograph for Professionals". Drugs.com. Archived from the original on 17 January 2021. Retrieved 25 October 2021.
- ↑ "Pamidronate (Aredia) Use During Pregnancy". Drugs.com. Archived from the original on 26 November 2020. Retrieved 25 October 2021.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 523. ISBN 9783527607495. Archived from the original on 2021-03-18. Retrieved 2021-04-22.
- ↑ "Pamidronate Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 14 May 2021. Retrieved 25 October 2021.
- ↑ I. Kubalek; O. Fain; J. Paries; A. Kettaneh; M. Thomas (2001). "Treatment of reflex sympathetic dystrophy with pamidronate: 29 cases". Rheumatology. 40 (12): 1394–1397. doi:10.1093/rheumatology/40.12.1394. PMID 11752511.
- ↑ Zarychanski R, Elphee E, Walton P, Johnston J (2006). "Osteonecrosis of the jaw associated with pamidronate therapy". Am J Hematol. 81 (1): 73–5. doi:10.1002/ajh.20481. PMID 16369966. S2CID 11830192.
- ↑ D., Tripathi, K. (2013-09-30). Essentials of medical pharmacology (Seventh ed.). New Delhi. ISBN 9789350259375. OCLC 868299888.
External links
| Identifiers: |
|---|