Extracellular polymeric substance
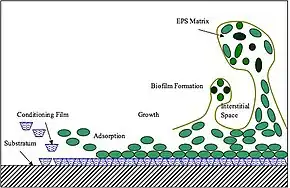
Extracellular polymeric substances (EPSs) are natural polymers of high molecular weight secreted by microorganisms into their environment.[1] EPSs establish the functional and structural integrity of biofilms, and are considered the fundamental component that determines the physicochemical properties of a biofilm.[2]
EPSs are mostly composed of polysaccharides (exopolysaccharides) and proteins, but include other macromolecules such as DNA, lipids and humic substances. EPSs are the construction material of bacterial settlements and either remain attached to the cell's outer surface, or are secreted into its growth medium. These compounds are important in biofilm formation and cells' attachment to surfaces. EPSs constitute 50% to 90% of a biofilm's total organic matter.[2][3][4]
Exopolysaccharides (also sometimes abbreviated EPSs; EPS sugars thereafter) are the sugar-based parts of EPSs. Microorganisms synthesize a wide spectrum of multifunctional polysaccharides including intracellular polysaccharides, structural polysaccharides and extracellular polysaccharides or exopolysaccharides. Exopolysaccharides generally consist of monosaccharides and some non-carbohydrate substituents (such as acetate, pyruvate, succinate, and phosphate). Owing to the wide diversity in composition, exopolysaccharides have found diverse applications in various food and pharmaceutical industries. Many microbial EPS sugars provide properties that are almost identical to the gums currently in use. With innovative approaches, efforts are underway to supersede the traditionally used plant and algal gums by their microbial counterparts. Moreover, considerable progress has been made in discovering and developing new microbial EPS sugars that possess novel industrial applications.[5] Levan produced by Pantoea agglomerans ZMR7 was reported to decrease the viability of rhabdomyosarcoma (RD) and breast cancer (MDA) cells compared with untreated cancer cells. In addition, it has high antiparasitic activity against the promastigote of Leishmania tropica.[6]
Function
Capsular exopolysaccharides can protect pathogenic bacteria against desiccation and predation, and contribute to their pathogenicity.[7] Sessile bacteria fixed and aggregated in biofilms are less vulnerable compared to drifting planktonic bacteria, as the EPS matrix is able to act as a protective diffusion barrier.[8] The physical and chemical characteristics of bacterial cells can be affected by EPS composition, influencing factors such as cellular recognition, aggregation, and adhesion in their natural environments.[8] Furthermore, the EPS layer acts as a nutrient trap, facilitating bacterial growth.[8]
The exopolysaccharides of some strains of lactic acid bacteria, e.g., Lactococcus lactis subsp. cremoris, contribute a gelatinous texture to fermented milk products (e.g., Viili), and these polysaccharides are also digestible.[9][10] An example of the industrial use of exopolysaccharides is the application of dextran in panettone and other breads in the bakery industry.[11]
Ecology
Exopolysaccharides can facilitate the attachment of nitrogen-fixing bacteria to plant roots and soil particles, which mediates a symbiotic relationship.[7] This is important for colonization of roots and the rhizosphere, which is a key component of soil food webs and nutrient cycling in ecosystems. It also allows for successful invasion and infection of the host plant.[7]
Bacterial extracellular polymeric substances can aid in bioremediation of heavy metals as they have the capacity to adsorb metal cations, among other dissolved substances.[12] This can be useful in the treatment of wastewater systems, as biofilms are able to bind to and remove metals such as copper, lead, nickel, and cadmium.[12] The binding affinity and metal specificity of EPSs varies, depending on polymer composition as well as factors such as concentration and pH.[12]
In a geomicrobiological context, EPSs have been observed to affect precipitation of minerals, particularly carbonates.[13] EPS may also bind to and trap particles in biofilm suspensions, which can restrict dispersion and element cycling.[13] Sediment stability can be increased by EPS, as it influences cohesion, permeability, and erosion of the sediment.[13] There is evidence that the adhesion and metal-binding ability of EPS affects mineral leaching rates in both environmental and industrial contexts.[13] These interactions between EPS and the abiotic environment allow for EPS to have a large impact on biogeochemical cycling.
Predator-prey interactions between biofilms and bacterivores, such as the soil-dwelling nematode Caenorhabditis elegans, had been extensively studied. Via the production of sticky matrix and formation of aggregates, Yersinia pestis biofilms can prevent feeding by obstructing the mouth of C. elegans.[14] Moreover, Pseudomonas aeruginosa biofilms can impede the slithering motility of C. elegans, termed as 'quagmire phenotype', resulting in trapping of C. elegans within the biofilms and preventing the exploration of nematodes to feed on susceptible biofilms.[15] This significantly reduced the ability of predator to feed and reproduce, thereby promoting the survival of biofilms.
Novel industrial use
Due to the growing need to find a more efficient and environmentally friendly alternative to conventional waste removal methods, industries are paying more attention to the function of bacteria and their EPS sugars in bioremediation.[16]
Researchers found that adding EPS sugars from cyanobacteria to wastewaters removes heavy metals such as copper, cadmium and lead.[16] EPS sugars alone can physically interact with these heavy metals and take them in through biosorption.[16] The efficiency of removal can be optimized by treating the EPS sugars with different acids or bases before adding them to wastewater.[16] Some contaminated soils contain high levels of polycyclic aromatic hydrocarbons (PAHs); EPSs from the bacterium Zoogloea sp. and the fungus Aspergillus niger, are efficient at removing these toxic compounds.[17] EPSs contain enzymes such as oxidoreductase and hydrolase, which are capable of degrading PAHs.[17] The amount of PAH degradation depends on the concentration of EPSs added to the soil. This method proves to be low cost and highly efficient.[17]
In recent years, EPS sugars from marine bacteria have been found to speed up the cleanup of oil spills.[18] During the Deepwater Horizon oil spill in 2010, these EPS-producing bacteria were able to grow and multiply rapidly.[18] It was later found that their EPS sugars dissolved the oil and formed oil aggregates on the ocean surface, which sped up the cleaning process.[18] These oil aggregates also provided a valuable source of nutrients for other marine microbial communities. This let scientists modify and optimize the use of EPS sugars to clean up oil spills.[18]
List of Extracellular polymeric substances
.svg.png.webp)
- acetan (Acetobacter xylinum)
- alginate (Azotobacter vinelandii)
- cellulose (Acetobacter xylinum)
- chitosan (Mucorales spp.)
- curdlan (Alcaligenes faecalis var. myxogenes)
- cyclosophorans (Agrobacterium spp., Rhizobium spp. and Xanthomonas spp.)
- dextran (Leuconostoc mesenteroides, Leuconostoc dextranicum and Lactobacillus hilgardii)
- emulsan (Acinetobacter calcoaceticus)
- galactoglucopolysaccharides (Achromobacter spp., Agrobacterium radiobacter, Pseudomonas marginalis, Rhizobium spp. and Zooglea' spp.)
- galactosaminogalactan (Aspergillus spp.)
- gellan (Aureomonas elodea and Sphingomonas paucimobilis)
- glucuronan (Sinorhizobium meliloti)
- N-acetylglucosamine (Staphylococcus epidermidis)
- N-acetyl-heparosan (Escherichia coli)
- hyaluronic acid (Streptococcus equi)
- indican (Beijerinckia indica)
- kefiran (Lactobacillus hilgardii)
- lentinan (Lentinus elodes)
- levan (Alcaligenes viscosus, Zymomonas mobilis, Bacillus subtilis)
- pullulan (Aureobasidium pullulans)
- scleroglucan (Sclerotium rolfsii, Sclerotium delfinii and Sclerotium glucanicum)
- schizophyllan (Schizophylum commune)
- stewartan (Pantoea stewartii subsp. stewartii)
- succinoglycan (Alcaligenes faecalis var. myxogenes, Sinorhizobium meliloti)
- xanthan (Xanthomonas campestris)
- welan (Alcaligenes spp.)
See also
- Extracellular matrix in multi-cellular organisms
- Exopolymer
- Sea snot
References
- ↑ Staudt C, Horn H, Hempel DC, Neu TR (2004). "Volumetric measurements of bacterial cells and extracellular polymeric substance glycoconjugates in biofilms". Biotechnol. Bioeng. 88 (5): 585–92. doi:10.1002/bit.20241. PMID 15470707.
- 1 2 Flemming, Hans-Curt; Wingender, Jost; Griebe, Thomas; Mayer, Christian (December 21, 2000), "Physico-Chemical Properties of Biofilms", in L. V. Evans (ed.), Biofilms: Recent Advances in their Study and Control, CRC Press, p. 20, ISBN 978-9058230935
- ↑ Donlan, Rodney M. (September 2002). "Biofilms: Microbial Life on Surfaces". Emerging Infectious Diseases. 8 (9): 881–890. doi:10.3201/eid0809.020063. PMC 2732559. PMID 12194761.
- ↑ Donlan RM, Costerton JW (2002). "Biofilms: survival mechanisms of clinically relevant microorganisms". Clin. Microbiol. Rev. 15 (2): 167–93. doi:10.1128/CMR.15.2.167-193.2002. PMC 118068. PMID 11932229.
- ↑ Suresh and Mody (2009). "Microbial Exopolysaccharides: Variety and Potential Applications". Microbial Production of Biopolymers and Polymer Precursors. Caister Academic Press. ISBN 978-1-904455-36-3.
- ↑ Al-Qaysi, Safaa A. S.; Al-Haideri, Halah; Al-Shimmary, Sana M.; Abdulhameed, Jasim M.; Alajrawy, Othman I.; Al-Halbosiy, Mohammad M.; Moussa, Tarek A. A.; Farahat, Mohamed G. (2021-05-28). "Bioactive Levan-Type Exopolysaccharide Produced by Pantoea agglomerans ZMR7: Characterization and Optimization for Enhanced Production". Journal of Microbiology and Biotechnology. 31 (5): 696–704. doi:10.4014/jmb.2101.01025. ISSN 1017-7825. PMID 33820887.
- 1 2 3 Ghosh, Pallab Kumar; Maiti, Tushar Kanti (2016). "Structure of Extracellular Polysaccharides (EPS) Produced by Rhizobia and their Functions in Legume–Bacteria Symbiosis: — A Review". Achievements in the Life Sciences. 10 (2): 136–143. doi:10.1016/j.als.2016.11.003.
- 1 2 3 Harimawan, Ardiyan; Ting, Yen-Peng (October 2016). "Investigation of extracellular polymeric substances (EPS) properties of P. aeruginosa and B. subtilis and their role in bacterial adhesion". Colloids and Surfaces B: Biointerfaces. 146: 459–467. doi:10.1016/j.colsurfb.2016.06.039. PMID 27395039.
- ↑ Welman AD (2009). "Exploitation of Exopolysaccharides from lactic acid bacteria". Bacterial Polysaccharides: Current Innovations and Future Trends. Caister Academic Press. ISBN 978-1-904455-45-5.
- ↑ Ljungh A, Wadstrom T (editors) (2009). Lactobacillus Molecular Biology: From Genomics to Probiotics. Caister Academic Press. ISBN 978-1-904455-41-7.
{{cite book}}:|author=has generic name (help) - ↑ Ullrich M, ed. (2009). Bacterial Polysaccharides: Current Innovations and Future Trends. Caister Academic Press. ISBN 978-1-904455-45-5.
- 1 2 3 Pal, Arundhati; Paul, A. K. (March 2008). "Microbial extracellular polymeric substances: central elements in heavy metal bioremediation". Indian Journal of Microbiology. 48 (1): 49–64. doi:10.1007/s12088-008-0006-5. PMC 3450203. PMID 23100700.
- 1 2 3 4 Tourney, Janette; Ngwenya, Bryne T. (2014-10-29). "The role of bacterial extracellular polymeric substances in geomicrobiology". Chemical Geology. 386 (Supplement C): 115–132. Bibcode:2014ChGeo.386..115T. doi:10.1016/j.chemgeo.2014.08.011.
- ↑ Atkinson, Steve; Goldstone, Robert J.; Joshua, George W. P.; Chang, Chien-Yi; Patrick, Hannah L.; Cámara, Miguel; Wren, Brendan W.; Williams, Paul (6 January 2011). "Biofilm Development on Caenorhabditis elegans by Yersinia Is Facilitated by Quorum Sensing-Dependent Repression of Type III Secretion". PLOS Pathogens. 7 (1): e1001250. doi:10.1371/journal.ppat.1001250. PMC 3017118. PMID 21253572.
- ↑ Chan, Shepherd Yuen; Liu, Sylvia Yang; Seng, Zijing; Chua, Song Lin (21 September 2020). "Biofilm matrix disrupts nematode motility and predatory behavior". The ISME Journal. 15 (1): 260–269. doi:10.1038/s41396-020-00779-9. PMC 7852553. PMID 32958848.
- 1 2 3 4 Mota, Rita; Rossi, Federico; Andrenelli, Luisa; Pereira, Sara Bernardes; De Philippis, Roberto (September 2016). "Released polysaccharides (RPS) from Cyanothece sp. CCY 0110 as biosorbent for heavy metals bioremediation: interactions between metals and RPS binding sites". Applied Microbiology and Biotechnology. 100 (17): 7765–7775. doi:10.1007/s00253-016-7602-9. PMID 27188779. S2CID 15287887.
- 1 2 3 Jia, Chunyun; Li, Peijun; Li, Xiaojun; Tai, Peidong; Liu, Wan; Gong, Zongqiang (2011-08-01). "Degradation of pyrene in soils by extracellular polymeric substances (EPS) extracted from liquid cultures". Process Biochemistry. 46 (8): 1627–1631. doi:10.1016/j.procbio.2011.05.005.
- 1 2 3 4 Gutierrez, Tony; Berry, David; Yang, Tingting; Mishamandani, Sara; McKay, Luke; Teske, Andreas; Aitken, Michael D. (27 June 2013). "Role of Bacterial Exopolysaccharides (EPS) in the Fate of the Oil Released during the Deepwater Horizon Oil Spill". PLOS ONE. 8 (6): e67717. Bibcode:2013PLoSO...867717G. doi:10.1371/journal.pone.0067717. PMC 3694863. PMID 23826336.