FCER1

| High-affinity IgE receptor; alpha | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | FCER1A | ||||||
| Alt. symbols | FcεRIα, FCE1A | ||||||
| NCBI gene | 2205 | ||||||
| HGNC | 3609 | ||||||
| OMIM | 147140 | ||||||
| RefSeq | NM_002001 | ||||||
| UniProt | P12319 | ||||||
| Other data | |||||||
| Locus | Chr. 1 q23 | ||||||
| |||||||
| High affinity IgE receptor; beta | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | MS4A2 | ||||||
| Alt. symbols | FcεRIβ, FCER1B, IGER, APY | ||||||
| NCBI gene | 2206 | ||||||
| HGNC | 7316 | ||||||
| OMIM | 147138 | ||||||
| RefSeq | NM_000139 | ||||||
| UniProt | Q01362 | ||||||
| Other data | |||||||
| Locus | Chr. 1 q23 | ||||||
| |||||||
| High affinity IgE receptor; gamma | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | FCER1G | ||||||
| Alt. symbols | FcεRIγ | ||||||
| NCBI gene | 2207 | ||||||
| HGNC | 3611 | ||||||
| OMIM | 147139 | ||||||
| RefSeq | NM_004106 | ||||||
| UniProt | P30273 | ||||||
| Other data | |||||||
| Locus | Chr. 1 q23 | ||||||
| |||||||
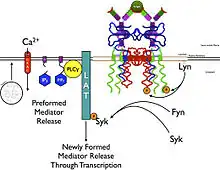
The high-affinity IgE receptor, also known as FcεRI, or Fc epsilon RI, is the high-affinity receptor for the Fc region of immunoglobulin E (IgE), an antibody isotype involved in the allergy disorder and parasites immunity. FcεRI is a tetrameric receptor complex that binds Fc portion of the ε heavy chain of IgE.[1] It consists of one alpha (FcεRIα - antibody binding site), one beta (FcεRIβ - which amplifies the downstream signal), and two gamma chains (FcεRIγ - the site where the downstream signal initiates) connected by two disulfide bridges on mast cells and basophils. It lacks the beta subunit on other cells. It is constitutively expressed on mast cells and basophils[2] and is inducible in eosinophils.
Tissue distribution
FcεRI is found on epidermal Langerhans cells, eosinophils, mast cells, and basophils.[3][4][5] As a result of its cellular distribution, this receptor plays a major role in controlling allergic responses. FcεRI is also expressed on antigen-presenting cells, and controls the production of important immune mediators (cytokines, interleukins, leukotrienes, and prostaglandins) that promote inflammation.[6] The most known mediator is histamine, which results in the five symptoms of inflammation: heat, swelling, pain, redness and loss of function.
FcεRI was demonstrated in bronchial/tracheal airway smooth muscle cells in normal and asthmatic patients. FcεRI cross-linking by IgE and anti-IgE antibodies led to Th2 (IL-4, -5, and -13) cytokines and CCL11/eotaxin-1 chemokine release; and ([Ca2+]i) mobilization, suggesting a likely IgE-FcεRI-ASM-mediated link to airway inflammation and AHR.[7][8]
Mechanism of action
Crosslinking of the FcεRI via IgE-antigen complexes leads to degranulation of mast cells or basophils and release of inflammatory mediators.[9] Under laboratory conditions, degranulation of isolated basophils can also be induced with antibodies to the FcεRIα, which crosslink the receptor. Such crosslinking and potentially pathogenic autoantibodies to the FcεRIα have been isolated from human cord blood, which suggest that they occur naturally and are present already at birth. However, their epitope on FcεRIα was masked by IgE, and the affinity of the corresponding autoantibodies found in healthy adults appeared lowered.[10]
See also
References
- ↑ Kumar, Vinay; Abbas, Abul K.; Aster, Jon (2012-05-01). Robbins Basic Pathology (9 ed.). Saunders.
- ↑ Pawankar R (February 2001). "Mast cells as orchestrators of the allergic reaction: the IgE-IgE receptor mast cell network". Current Opinion in Allergy and Clinical Immunology. 1 (1): 3–6. doi:10.1097/00130832-200102000-00002. PMID 11964662.
- ↑ Ochiai K, Wang B, Rieger A, Kilgus O, Maurer D, Födinger D, Kinet J, Stingl G, Tomioka H (1994). "A review on Fc epsilon RI on human epidermal Langerhans cells". International Archives of Allergy and Immunology. 104. Suppl 1 (1): 63–64. doi:10.1159/000236756. PMID 8156009.
- ↑ Prussin C, Metcalfe D (1994). "5". Nature. 367 (6459): 183–6. doi:10.1038/367183a0. PMID 8114916. S2CID 4331405.
- ↑ Gounni, A.; Lamkhioued, B.; Ochiai, K.; Tanaka, Y.; Delaporte, E.; Capron, A.; Kinet, J.P.; Capron, M. (2006). "IgE, mast cells, basophils, and eosinophils". Journal of Allergy and Clinical Immunology. 117 (2 Suppl Mini–Primer): S450–5456. doi:10.1016/j.jaci.2005.11.016. PMID 16455345.
- ↑ von Bubnoff D, Novak N, Kraft S, Bieber T (2003). "The central role of FcepsilonRI in allergy". Clinical and Experimental Dermatology. 28 (2): 184–187. doi:10.1046/j.1365-2230.2003.01209.x. PMID 12653710. S2CID 2080598.
- ↑ Gounni, A.S.; Wellemans, V.; Yang, J.; Bellesort, F.; Kassiri, K.; Gangloff, S.; Guenounou, M.; Halayko, A.J.; Hamid, Q.; Lamkhioued, B. (August 15, 2005). "Human airway smooth muscle cells express the high affinity receptor for IgE (Fc epsilon RI): a critical role of Fc epsilon RI in human airway smooth muscle cell function". Journal of Immunology. 175 (4): 2613–2621. doi:10.4049/jimmunol.175.4.2613. PMID 16081836.
- ↑ Gounni, A.S. (September 2006). "The high-affinity IgE receptor (FcepsilonRI): a critical regulator of airway smooth muscle cells?". American Journal of Physiology. 291 (3): L312-321. doi:10.1152/ajplung.00005.2006. PMID 16581830.
- ↑ Siraganian RP (December 2003). "Mast cell signal transduction from the high-affinity IgE receptor". Current Opinion in Immunology. 15 (6): 639–646. doi:10.1016/j.coi.2003.09.010. PMID 14630197.
- ↑ Bobrzynski T, Fux M, Vogel M, Stadler MB, Stadler BM, Miescher SM (November 2005). "A high-affinity natural autoantibody from human cord blood defines a physiologically relevant epitope on the FcepsilonRIalpha". Journal of Immunology. 175 (10): 6589–6596. doi:10.4049/jimmunol.175.10.6589. PMID 16272313.
External links
- Fc+epsilon+RI at the US National Library of Medicine Medical Subject Headings (MeSH)