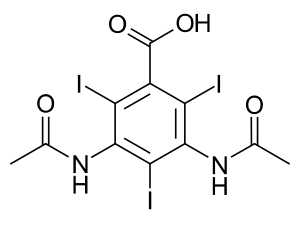
Diatrizoate
 | |
 | |
| Names | |
|---|---|
| Trade names | Hypaque, Gastrografin, Iothalmate, Urografin, others |
| Other names | amidotrizoic acid, diatrizoic acid, 3,5-diacetamido-2,4,6-triiodobenzoic acid |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Defined daily dose | not established[1] |
| External links | |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Chemical and physical data | |
| Formula | C11H9I3N2O4 |
| Molar mass | 613.916 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Diatrizoate, also known as amidotrizoate, is a contrast agent used during X-rays.[2] This includes when visualizing veins, the urinary system, spleen, and joints, as well as during computer tomography (CT scan).[2] It is given by mouth, injection into a vein, injection into the bladder, through a nasogastric tube, or rectally.[3][4]
Relatively common side effects include vomiting, diarrhea, and skin redness.[5] Other side effects include itchiness, kidney problems, low blood pressure, and allergic reactions.[2] It is not recommended in people who have an iodine allergy.[2] Diatrizoate is an iodinated ionic radiocontrast agent with high osmolality.[3]
Diatrizoate was approved for medical use in the United States in 1954.[5] It is on the World Health Organization's List of Essential Medicines.[6] As of 2016, the wholesale cost in the developing world is about US$5.49 per 20 ml vial.[7] In the United States a dose costs less than US$25.[3]
Medical uses

Diatrizoic acid may be used as an alternative to barium sulfate for medical imaging of the gastrointestinal tract, such as upper gastrointestinal series and small bowel series. It is indicated for use in patients who are allergic to barium, or in cases where the barium might leak into the abdominal cavity. It does not coat the stomach/bowel lining as well as barium, so it is not used commonly for this purpose.
It is used for intravenous pyelography.
It is also used to treat Ascaris lumbricoides (roundworms).[8][9] Diatrizoate may not actually kill Ascaris, but instead it promotes shifting of fluid in the bowel lumen, so may relieve intestinal obstruction caused by impacted Ascaris.[10]
Administration
In principle, diatrizoic acid is administered by the route most appropriate and sensible to image the structure/-s of interest (e.g., IV for blood vessels through which it is distributed and kidney–ureters–bladder that excrete it; orally or per rectally as an enema for the gastrointestinal tract).
- It is given intravenously to enhance contrast in computed tomography, to image the kidneys and related structures, and to image blood vessels.
- It is given orally or by enema to image the gastrointestinal tract.
- It is given by Foley catheter to image the urinary tract.
Dosage
The defined daily dose is not established[1]
Contraindications
A history of sensitivity to iodine is not a contraindication to using diatrizoate, although it suggests caution in use of the agent. In this case, a regimen of oral or intravenous corticosteroids may be given as prophylaxis, or an alternative such as barium sulfate may be preferable.
Gastrografin is contraindicated to use along with certain medications that can cause lactic acidosis, such as metformin. Concurrent use may lead to kidney failure and lactic acidosis, and a clinician may need to space the agents apart over a number of days to prevent an interaction.[11]
Gastrografin is a hypertonic solution, and therefore it should be avoided in imaging studies of the upper gastrointestinal tract in patients who are at risk of aspiration, as it will cause prompt pulmonary edema if accidentally introduced into the tracheobronchial tree.
Urografin is not to be used for myelography, ventriculography or cisternography, since it is likely to provoke neurotoxic symptoms in these examinations. [12]
Chemistry
Diatrizoate is considered a high-osmolality contrast agent. Its osmolality ranges from approximately 1500 mOsm/kg (50% solution)[13] to over 2000 mOsm/kg (76% solution).[14]
Brand names
Brand names include Hypaque, Gastrografin, MD-Gastroview, Iothalmate, and Urografin. Urografin is a combination of the sodium and meglumine salts.
See also
The core structure is a popular one and numerous analogues exist.
- Mono-compounds
- Acetrizoic acid and its sodium salt sodium acetrizoate
- Metrizoic acid
- Iodamide
- Iotalamic acid
- Ioxitalamic acid
- Ioglicic acid
- Iocetamic acid
- Bis-compounds
References
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 1 July 2021. Retrieved 15 September 2020.
- 1 2 3 4 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 316. hdl:10665/44053. ISBN 9789241547659.
- 1 2 3 Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 171. ISBN 9781284057560.
- ↑ Thomsen, Henrik; Muller, Robert N.; Mattrey, Robert F. (2012). Trends in Contrast Media. Springer Science & Business Media. p. 13. ISBN 9783642598142. Archived from the original on 2017-01-01.
- 1 2 "Diatrizoate Side Effects in Detail - Drugs.com". www.drugs.com. Archived from the original on 1 January 2017. Retrieved 31 December 2016.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Amidotrizoate". International Drug Price Indicator Guide. Archived from the original on 29 March 2019. Retrieved 8 December 2016.
- ↑ Sood. Surgical Diseases in Tropical Countries. Jaypee Brothers Publishers. pp. 72–. ISBN 978-81-7179-444-7. Retrieved 11 August 2010.
{{cite book}}: CS1 maint: url-status (link) - ↑ Moshe Schein; Paul Rogers; Ahmad Assalia (2010). Schein's Common Sense Emergency Abdominal Surgery. Springer. pp. 391–. ISBN 978-3-540-74820-5. Archived from the original on 29 March 2017. Retrieved 11 August 2010.
- ↑ "Archived copy". Archived from the original on 2016-06-18. Retrieved 2016-07-28.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Micromedex Healthcare Series (November 2010). "DIATRIZOATE MEGLUMINE/DIATRIZOATE SODIUM". DRUGDEX. Thomson Reuters (Healthcare) Inc. Retrieved 2010-11-10.
- ↑ Bayer New Zealand Limited (September 2007). "UROGRAFIN Urografin Corporate Core Text" (PDF). Archived (PDF) from the original on 2016-03-04. Retrieved 2012-04-02.
- ↑ Amersham Health (April 2006). "Hypaque sodium (Diatrizoate Sodium) injection, solution. Product label". DailyMed. U.S. National Library of Medicine. Archived from the original on 2011-05-23. Retrieved 2007-03-29.
- ↑ Amersham Health (April 2006). "Hypaque (Diatrizoate Meglumine and Diatrizoate Sodium) injection, solution. Product label". DailyMed. U.S. National Library of Medicine. Archived from the original on 2011-05-23. Retrieved 2007-03-29.
External links
| External sites: |
|
|---|---|
| Identifiers: |