Glyceroneogenesis
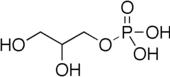
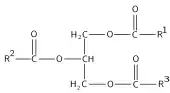
Glyceroneogenesis is a metabolic pathway which synthesizes glycerol 3-phosphate or triglyceride from precursors other than glucose.[1] Usually glycerol 3-phosphate is generated from glucose by glycolysis, but when glucose concentration drops in the cytosol, it is generated by another pathway called glyceroneogenesis. Glyceroneogenesis uses pyruvate, alanine, glutamine or any substances from the TCA cycle as precursors for glycerol 3-phosphate. Phosphoenolpyruvate carboxykinase (PEPC-K),[1] which is an enzyme that catalyzes the decarboxylation of oxaloacetate to phosphoenolpyruvate is the main regulator for this pathway. Glyceroneogenesis can be observed in adipose tissue and also liver. It is a significant biochemical pathway which regulates cytosolic lipid levels. Intense suppression of glyceroneogenesis may lead to metabolic disorder such as type 2 diabetes.[2]
Summary
In mammals, triglycerol or its backbone, glycerol 3- phosphate, is usually synthesized from glucose through glycolysis.[1] Glucose will be degraded though glycolysis until fructose 1,6-bisphosphate is broken down to glyceraldehyde 3-phosphate and dihydroxyacetone phosphate. Dihydroxyacetone phosphate is significant in synthesizing triglycerides because it can be used to generate glycerol 3-phosphate. However, glycerol 3-phosphate is generated through a different pathway when an organism is deficient of carbohydrates such as glucose. During fasting or low carbohydrate diet, glycerol 3-phosphate is generated by another metabolic pathway called glyceroneogenesis which uses precursors other than glucose. Glyceroneogenesis is considerably important because it is the dominant pathway to generate lipids during fasting or starvation. Not only does it produce lipids for the organism but it also regulates lipid levels in the cell.[1] Glyceroneogenesis involves re-esterification of fatty acids to generate triglycerides. In other words, it can regulate fatty acid concentration in the cytosol. Strong activity in glyceroneogenesis will induce the re-esterification of fatty acid which will result in decrease of fatty acid concentration in the cytosol. Therefore, glyceroneogenesis is significantly related to lipid control of mammals.
Metabolic pathway
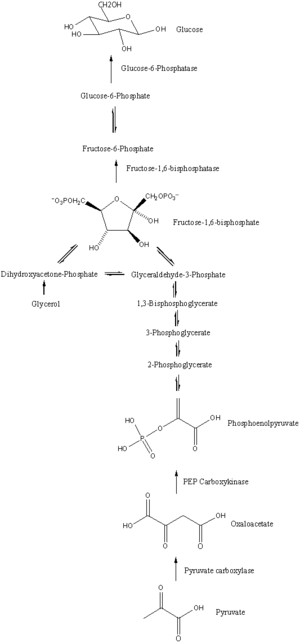

The main precursors of glyceroneogenesis are pyruvate, lactate, glutamine, and alanine. Glyceroneogenesis is also known as branched pathway of gluconeogenesis because the first few steps in glyceroneogenesis are exactly the same as gluconeogenesis (Figure 3).
When pyruvate or lactate is used as the precursor for glycerol 3-phosphate, glyceroneogenesis follows exactly the same pathway as gluconeogenesis until it generates dihydroxyacetone phosphate. Lactate catalysed by lactate dehydrogenase will form pyruvate with expense of NAD+. Furthermore, by using 1 ATP and bicarbonate, pyruvate will be converted to oxaloacetate. which is catalysed by pyruvate carboxylase. Oxaloacetate will be catalysed by PEPC-K to generate phosphoenolpyruvate. This phosphorylation and decarboxylation of oxaloacetate is the significant step in glyceroneogenesis because the entire pathway is regulated by this reaction. After the production of phosphoenolpyruvate, gluconeogenesis will continue until dihydroxyacetone phosphate is generated, which produces 2-phosphoglycerate, 3-phosphoglycerate, 1,3-bisphosphoglycerate and glyceraldehyde 3-phosphate as intermediates. When dihydroxyacetone phosphate is produced, glyceroneogenesis will branch off from gluconeogenesis.[1] With expense of NADH, dihydroxyacetone phosphate will convert to glycerol 3- phosphate (Figure 4), which is the final product of glyceroneogenesis. In addition, triglyceride can be generated by re-esterification of 3 fatty acid chains on glycerol 3-phosphate. Therefore, glyceroneogenesis is a metabolic pathway starting from lactate or pyruvate, and it is similar to gluconeogenesis but the pathway will branch out when dihydroxyacetone phosphate is generated. Instead of producing fructose 1,6- bisphosphate as gluconeogenesis does, glyceroneogenesis converts dihydroxyacetone phosphate to glycerol 3-phosphate.
Alanine can also be used as a precursor of glyceroneogenesis because alanine can be degraded to pyruvate. Alanine will degrade to pyruvate by transferring its amino group to 2-oxoglutarate with an enzyme called alanine aminotransferase. Alanine aminotransferase will cleave off the amino group from alanine and binds it to 2-oxoglutarate which generates pyruvate from alanine and glutamate from 2-oxoglutarate. Pyruvate generated from alanine will enter glyceroneogenesis and generate glycerol 3-phosphate.
Glutamate is also a known metabolite substance which can enter glyceroneogenesis. Since the key reaction of glyceroneogenesis is the decarboxylation and phosphorylation of oxaloacetate to phosphoenolpyruvate, in theory, any biochemical pathway which generates oxaloacetate is related to glyceroneogenesis. For example, glutamate can generate oxaloacetate in 2 steps. First of all, glutamate can be converted to 2-oxoglutarate with expense of NAD+ and H2O with help of glutamate dehydrogenase. Secondly, 2-oxoglutarate can enter tricarboxyl acid cycle in order to generate oxaloacetate. Therefore, theoretically any metabolites in the TCA cycle or any metabolites generating the metabolites of TCA cycle can be used as a precursor of glyceroneogenesis, but glutamate is the only precursor confirmed,
Regulation

Phosphoenolpyruvate carboxykinase (PEPC-K)
Glyceroneogenesis can be regulated at two reaction pathways. First of all, it can be regulated at the decarboxylation of oxaloacetate to phosphoenolpyruvate. Secondly, TCA cycle can affect glyceroneogenesis when the glutamate or substrates in TCA cycle are being used as a precursor. Decarboxylation of oxaloacetate to phosphoenolpyruvate is catalysed by enzyme PEPC-K.[1] PEPC-K is known as the essential enzyme which regulates glyceroneogenesis. Increasing the amount of PEPC-K or over expressing the gene for PEPC-K will increase the activity of glyceroneogenesis. More oxaloacetate can be decarboxylated to phosphoenolpyruvate when there is more PEPC-K that can catalyse the reaction. Furthermore, gene expression of PEPC-K can be suppressed by hormones named norepinephrine, glucocorticoid, and insulin.[3] Norepinephrine is a neurotransmitter hormone which decreases the activity of PEPC-K when the cell is oriented in a cold environment. As a result, glyceroneogenesis is more likely to decrease in activity in cold environment. Glucocorticoid is a steroid hormone which is involved in the reciprocal regulation of glyceroneogenesis in liver and adipose tissues. Unfortunately, the actual mechanism of the reciprocal regulation is not well understood, but glucocorticoids induce transcription of PEPC-K in liver while decreasing the transcription in adipose tissues. Insulin is a peptide hormone which induces cells to intake glucose. In glyceroneogenesis, insulin down regulates the expression of PEPC-K in both liver and adipose tissues.
TCA cycle
When metabolites from TCA cycle or glutamate are used as a precursor for glyceroneogenesis, the regulator in the TCA cycle can also cause flux to products formed by glyceroneogenesis. Regulation of TCA cycle is mostly determined by product inhibition and substrate availability. TCA cycle will slow down when excess product is present in the environment or deficient of substrate such as ADP and NAD+
Location
Since glyceroneogenesis is related to lipid regulation, it can be found in adipose tissue and liver. In adipose tissue, glyceroneogenesis restrains the release of free fatty acids by re-esterifying them and in liver, triglycerides are being synthesized for lipid distribution.
White adipose tissue
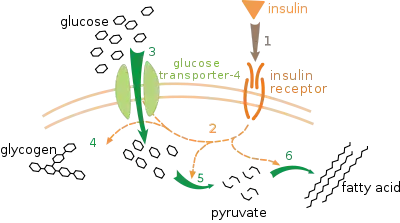
White adipose tissue, also known as white fat, is one of the 2 types of adipose tissue in mammals. White adipose tissue stores energy in form of triglycerides that can be broken down to free fatty acids. Its normal function is to store free fatty acids as triglycerides within the tissue. However, when the glucose level of in the cell drops in situations like fasting, white adipose tissue generates glycerol 3-phosphate.[3] The presence of glyceroneogenesis in white adipose tissues is proven by an experiment with mouse.[1] Since glycerol 3-phosphate is usually generated from glucose through glycolysis, level of triglyceride content was compared with normal mouse and mouse which cannot intake glucose in to their cells. Glucose transporter 4, also known as GLUT 4 (Figure 6) is the transporter protein of glucose which intakes extracellular glucose to intracellular environment. In order to examine the presence of glyceroneogenesis in mouse, genes expressing GLUT4 were deleted and triglyceride content of adipose tissue was compared with normal mouse. Since glucose cannot enter the cell, synthesis of glycerol 3-phosphate was expected to decrease. However, the result showed no change in triglyceride concentration. This experiment proved the presence of an alternate metabolic pathway to synthesize triglyceride in adipose tissues of mouse.[1] Furthermore, an additional experiment was performed in order to examine the relation of the alternate glycerol 3-phosphate synthesizing pathway and PEPC-K. Triglyceride content in white adipose tissue of mouse with mutated gene which expresses PEPC-K was observed. Sine PEPC-K is the essential regulatory enzyme for glyceroneogenesis, mutation in PEPC-K genes would lower the activity of glyceroneogenesis. The result showed no production of triglyceride in white adipose tissues as expected.[3] Hence, glyceroneogenesis was present in white adipose tissues because it was able to generate triglycerides without glucose and it was unable to synthesize when PEPC-K was mutated. Therefore, during fasting or low carbohydrate diet, white adipose tissues generate glycerol 3-phosphate using glyceroneogenesis.
Brown adipose tissue
Brown adipose tissue is another type of adipose tissue which stores free fatty acids. Brown adipose tissue is especially abundant in new born mammals and hibernating mammals. The differences between brown and white adipose tissue are brown adipose tissues have higher activity in glyceroneogenesis than white adipose tissues and glyceroneogenesis in brown adipose tissue is related to thermogenesis.[3] The activity of glyceroneogenesis in brown adipose tissue is greater than that of white adipose tissue because it contains more enzymes involved in the glyceroneogenesis. Compared to white adipose tissue, brown adipose tissue has considerably higher activity of PEPC-K and glycerol kinase. The activity of PEPC-K in brown adipose tissue is almost 10 times the activity of that in white adipose tissue.[3] PEPC-K which is involved in the conversion of oxaloacetate to phosphoenolpyruvate is the key enzyme that regulates glyceroneogenesis. Increase in activity of the enzyme will increase the activity of the pathway. Furthermore, not only PEPC-K but brown adipose tissue is also rich in activity of glycerol kinase. Glycerol kinase is the enzyme which phosphorylates glycerol in order to generate the backbone of triglycerides, glycerol 3-phosphate. Increase in the activity of glycerol kinase will result in an increase in production of glycerol 3- phosphate. As a result, brown adipose tissue will have greater activity in glyceroneogenesis, because it contains more enzymes involved in the pathway.
In addition, glyceroneogenesis in brown adipose tissue is related to thermogenesis in the organism. In mammals, heat is generated by delivering free fatty acids to the mitochondria.[3] When glyceroneogenesis is proceeding regularly, the concentration of free fatty acid is low in intercellular environment because glyceroneogenesis re-esterifies fatty acids to triglycerides. In other words, thermogenesis by free fatty acids is less likely to occur when glyceroneogenesis is proceeding. However, when exposed to cold, a neurotransmitter hormone called norepinephrine will suppress the activity of PEPC-K.[3] When the activity of PEPC-K is suppressed, glyceroneogenesis will be unable to re-esterify the free fatty acids. Eventually, the free fatty acid concentration within the cell will increase leading to excessive free fatty acids in cytosol, which will consequently be delivered to the mitochondria for thermogenesis.[4] Therefore, when a mammal is exposed to cold, heat is generated in the brown adipose tissue by decreasing the activity of glyceroneogenesis.
Liver
Although glyceroneogenesis was first found in adipose tissues, it was not recognized in the liver until 1998 (Source?). Glyceroneogenesis was unexpected in the liver for 2 reasons; triglyceride synthesis in liver was thought to be not natural because gluconeogenesis is taking huge part in the liver, and liver was believed to have sufficient glycerol 3-phosphate collected from the bloodstream. However, several experiments, which used stable isotopes to track the glycerol in liver and bloodstream, showed that 65% of the glycerol backbone of triglyceride flowing through the bloodstream is actually synthesised in the liver.[3] Therefore, glycerol 3-phosphate synthesis in liver was discovered. In fact, liver synthesizes more than half of the glycerol which mammals need to regulate lipid in their body.
Glyceroneogenesis in liver and adipose tissues regulate lipid metabolism in opposite ways. On one hand, lipids in the form of triglycerides are released from the liver. However, on the other hand, glyceroneogenesis restrains the fatty acid release from adipose tissues by re-esterifying them. In other words, glyceroneogenesis in liver and adipose tissues are alternately regulated.[3] When the lipid concentration in the blood is relatively high, glyceroneogenesis in liver will negatively regulated to stop the synthesis of triglyceride, but glyceroneogenesis in adipose tissues will be induced in order to restrain the release of free fatty acid to the bloodstream. Conversely, glyceroneogenesis in liver will be induced and suppressed in adipose tissues when lipid level of the blood is low. Even though the reciprocal regulation of glyceroneogenesis is not well understood, a hormone called glucocorticoid is a best example of the regulation.[4] Glucocorticoids induce gene transcription of PEPC-K in liver but repress the transcription in adipose tissues.
Disease
Type 2 Diabetes

Failure in regulating glyceroneogenesis may lead to Type 2 diabetes.[5] Type 2 diabetes is a metabolic disorder that results in high levels of blood glucose and blood lipid. Type2 diabetes is associated with over production of triglycerides in liver due to excessively active glyceroneogenesis and excess release of fatty acids from adipose tissues. Since the activity of glyceroneogenesis is mostly dependent on PEPC-K, fluctuating the expressions for PEPC-K will dramatically influence the activity of glyceroneogenesis. Over expressing PEPC-K in liver will eventually result in over production of triglycerides which can elevate the lipid level in the bloodstream. Conversely, in adipose tissue, down regulated glyceroneogenesis may decrease de novo lipogenesis. Suppressed glyceroneogenesis will result in increase of free fatty acids in the adipose tissues and its subsequent export to the bloodstream, because re-esterification of free fatty acids into triglycerides will decrease due to the decreased availability of the glycerol backbone. Therefore, glyceroneogenesis overly induced in liver and reduced in adipose tissues can lead, respectively, to hepatic steatosis and lipodystrophy, both highly associated to Type 2 Diabetes.
Treatment
Regulation of Glyceroneogenesis is a therapeutic target of Type II diabetes. The release of triglycerides in the liver should be inhibited as well as release of free fatty acid in adipose tissues. Insulin is used as a down regulator in liver of glyceroneogenesis. Suppression in glyceroneogenesis will decrease the triglyceride being released in to the bloodstream from liver. However, the problem with insulin is that it also suppresses glyceroneogenesis in adipose tissue. In order to restrict the release of free fatty acid from adipose tissues, fatty acids must be re-esterified by glyceroneogenesis. Thiazolidinedione (Figure 8) is a substance which only affects glyceroneogenesis in adipose tissue. Thiazolidinedione will increase the transcription of PEPC-K and eventually induce the activity of glyceroneogenesis.[5] As a result, re-esterification of fatty acids take place in the cell and prevents the release of fatty acids to the bloodstream.[5]
See also
References
- 1 2 3 4 5 6 7 8 Nye CK, Hanson RW, Kalhan SC (October 2008). "Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat". The Journal of Biological Chemistry. 283 (41): 27565–74. doi:10.1074/jbc.M804393200. PMC 2562054. PMID 18662986.
- ↑ Jeoung NH, Harris RA (October 2010). "Role of pyruvate dehydrogenase kinase 4 in regulation of blood glucose levels". Korean Diabetes Journal. 34 (5): 274–83. doi:10.4093/kdj.2010.34.5.274. PMC 2972486. PMID 21076574.
- 1 2 3 4 5 6 7 8 9 Reshef L, Olswang Y, Cassuto H, Blum B, Croniger CM, Kalhan SC, Tilghman SM, Hanson RW (August 2003). "Glyceroneogenesis and the triglyceride/fatty acid cycle". The Journal of Biological Chemistry. 278 (33): 30413–6. doi:10.1074/jbc.R300017200. PMID 12788931.
- 1 2 Chaves VE, Frasson D, Martins-Santos ME, Boschini RP, Garófalo MA, Festuccia WT, Kettelhut IC, Migliorini RH (October 2006). "Glyceroneogenesis is reduced and glucose uptake is increased in adipose tissue from cafeteria diet-fed rats independently of tissue sympathetic innervation". The Journal of Nutrition. 136 (10): 2475–80. doi:10.1093/jn/136.10.2475. PMID 16988112.
- 1 2 3 Beale EG, Hammer RE, Antoine B, Forest C (April 2004). "Disregulated glyceroneogenesis: PCK1 as a candidate diabetes and obesity gene". Trends in Endocrinology and Metabolism. 15 (3): 129–35. doi:10.1016/j.tem.2004.02.006. PMID 15046742.