Hycanthone
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.019.512 |
| Chemical and physical data | |
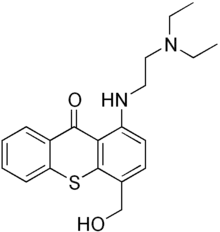
| Formula | C20H24N2O2S |
| Molar mass | 356.48 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Hycanthone is the schistosomicide approved by the FDA in 1975. It is a metabolite of lucanthone. Hycanthone interferes with parasite nerve function, resulting in paralysis and death. This agent also intercalates into DNA and inhibits RNA synthesis in vitro and shows potential antineoplastic activity.[1]
Anti-schistosomal activity
Hycanthone is shown to be an effective inhibitor of acetylcholinesterase (AChE) from Schistosoma mansoni, but is less potential against AChE from mammalian origin. This might come from differences in the configuration of active center between schistosome and mammalian AChE enzymes.[2]
Hycanthone is shown to intercalates into DNA and inhibit RNA synthesis in vitro. A growing body of evidence has shown that hycathone has an antineoplastic activity.
Clinical trials
Physical properties
| Physical state | Solid |
| Solubility | Soluble in ethanol, methanol, DMSO, and water |
| Absorption maximum | 233, 258, 329, 438 nm |
| Melting point | 173-176 °C |
| logP | 3.74 |
References
- ↑ "hycanthone". NCI Cancer Dictionary.
- ↑ Hillman GR, Senft AW (September 1975). "Anticholinergic properties of the antischistosomal drug hycanthone". The American Journal of Tropical Medicine and Hygiene. 24 (5): 827–34. doi:10.4269/ajtmh.1975.24.827. PMID 1190369.
- ↑ Schutt AJ, Dalton RJ, Kovach JS, Moertel CG, O'Connell MJ (June 1983). "Phase II study of hycanthone in patients with advanced colorectal carcinoma". Cancer Treatment Reports. 67 (6): 593–4. PMID 6861166.
- ↑ "Phase II Chemotherapy with Hycanthone Mesylate and Flagyl for Advanced Malignant Lymphomas". U.S. National Cancer Institute. Archived from the original on 13 February 2015.