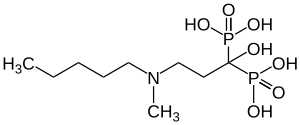
Ibandronic acid
 | |
| Names | |
|---|---|
| Trade names | Boniva, Bonviva, Bondronat, others |
| Other names | Ibandronate sodium |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, intravenous |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 0.6% |
| Protein binding | 90.9 to 99.5% (concentration-dependent) |
| Metabolism | Nil |
| Elimination half-life | 10 to 60 hours |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C9H23NO7P2 |
| Molar mass | 319.231 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ibandronic acid, also known as ibandronate, is a medication used treat of osteoporosis, high calcium due to cancer, and bone metastases from breast cancer.[1][2] It may be taken by mouth or injection into a vein.[1]
Common side effects include heart burn, low calcium, weakness, headache, and fever.[2] Other side effects may include anaphylaxis, esophagitis, femur fracture, and osteonecrosis of the jaw.[2][3] It is a bisphosphonate and works by stopping bone breakdown by cells known as osteoclasts.[1][2]
Ibandronic acid was patented in 1986 by Boehringer Mannheim and approved for medical use in 1996.[4] It is available as a generic medication.[1] In the United Kingdom a 150 mg pill cost the NHS about £4.50 as of 2021.[1] This amount in the United States is about 12 USD.[5]
Medical uses
Ibandronate is indicated for the treatment and prevention of osteoporosis in post-menopausal women.[6] The basis for this approval was a trial of women with post-menopausal osteoporosis. Every participant also received daily oral doses of calcium and 400IUs [international units] of vitamin D. At the study's conclusion, both doses significantly reduced the occurrence risk of new vertebral fractures by 50–52 percent when compared to the effects of the placebo drug.
Ibandronate is efficacious for the prevention of metastasis-related bone fractures in multiple myeloma, breast cancer, and certain other cancers.[7]
Dosage
For osteoporosis it is take by mouth at a dose of 150 mg per month or by intravenous at a dose of 3 mg every 3 month.[1]
Side effects
In 2008, the U.S Food and Drug Administration (FDA) issued a communication warning of the possibility of severe and sometimes incapacitating bone, joint or muscle pain.[8] A study conducted by the American Society of Bone and Mineral Research concluded that long-term use of bisphosphonates, including Boniva, may increase the risk of a rare but serious fracture of the femur. [9] The drug also has been associated with osteonecrosis of the jaw, relatively rare but serious condition.[10]
Pharmacology
| Bisphosphonate | Relative potency |
|---|---|
| Etidronate | 1 |
| Tiludronate | 10 |
| Pamidronate | 100 |
| Alendronate | 100-500 |
| Ibandronate | 500-1000 |
| Risedronate | 1000 |
| Zoledronate | 5000 |
Society and culture
Cost
This medication has a cost in the U.S. of $51 (USD) for 3 tablets (150 mg)[12]
.svg.png.webp) IbandronateSodium costs (US)
IbandronateSodium costs (US).svg.png.webp) IbandronateSodium prescriptions (US)
IbandronateSodium prescriptions (US)
Brand names
Ibandronic acid is marketed under the trade names Boniva in the USA, Bondronat in Europe, Bonviva in Asia, Bandrone in India, Ibandrix in Ecuador, Adronil in Pakistan, Bondrova in Bangladesh and Bonprove in Egypt.
References
- 1 2 3 4 5 6 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 769. ISBN 978-0857114105.
- 1 2 3 4 "Bondronat". Archived from the original on 11 January 2021. Retrieved 24 November 2021.
- ↑ "Ibandronate Monograph for Professionals". Drugs.com. Archived from the original on 11 December 2018. Retrieved 24 November 2021.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 523. ISBN 9783527607495. Archived from the original on 2021-03-18. Retrieved 2020-12-06.
- ↑ "Ibandronate Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 24 November 2021.
- ↑ "boniva". The American Society of Health-System Pharmacists. Archived from the original on 11 December 2018. Retrieved 3 April 2011.
- ↑ Sittig HB (2012). "Pathogenesis and bisphosphonate treatment of skeletal events and bone pain in metastatic cancer: focus on ibandronate". Onkologie. 35 (6): 380–7. doi:10.1159/000338947. PMID 22722461. S2CID 8413102.
- ↑ "Information for Healthcare Professionals: Bisphosphonates (marketed as Actonel, Actonel+Ca, Aredia, Boniva, Didronel, Fosamax, Fosamax+D, Reclast, Skelid, and Zometa)". U.S. Food and Drug Administration. Archived from the original on 22 October 2010. Retrieved 27 October 2010.
- ↑ "Drugs Commonly Prescribed for Osteoporosis Patients are Effective at Reducing Risk of Hip and Spine Fractures, But Panel Says May be Related to Unusual Thigh Bone Fractures When Used Long Term". Journal of Bone and Mineral Research=27 October 2010. Archived from the original on 9 April 2016. Retrieved 6 December 2020.
- ↑ "Osteonecrosis of the jaw (ONJ) and drug treatments for osteoporosis" (PDF). nos.org.uk. The National Osteoporosis Society. Archived from the original (PDF) on 2017-06-17. Retrieved 2020-12-06.
- ↑ Tripathi KD (2013-09-30). Essentials of medical pharmacology (Seventh ed.). New Delhi. ISBN 9789350259375. OCLC 868299888.
- ↑ "Ibandronate Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 12 November 2020. Retrieved 1 April 2021.
External links
| Identifiers: |
|---|
- "Ibandronic acid". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-11-17. Retrieved 2020-12-06.