Incentive spirometer
| Incentive spirometer | |
|---|---|
 A typical incentive spirometer consists of an inhalation nozzle, which is seen facing toward the camera. The curved plastic on the left is a handle. The plunger is in the middle (along with an adjustable mark to indicate a goal), and on the right side is a flow indicator showing whether the patient is inhaling too rapidly. | |
| Specialty | Pulmonology |
An incentive spirometer is a handheld medical device used to help patients improve the functioning of their lungs. By training patients to take slow and deep breaths, this simplified spirometer facilitates lung expansion and strengthening. Patients inhale through a mouthpiece, which causes a piston inside the device to rise. This visual feedback helps them monitor their inspiratory effort. Incentive spirometers are commonly used after surgery or other illnesses to prevent pulmonary complications.
Overview
Incentive spirometer is indicated for patients who have had any surgery that might jeopardize respiratory function, particularly surgery to the lungs,[1] but also to patients recovering from cardiac or other surgery involving extended time under anesthesia and prolonged in-bed recovery. Under general anesthesia and inactivity, a patient's breathing may slow, causing air sacs in their lungs to not fully inflate. Atelectasis can develop and, if unmanaged, lead to pneumonia and postoperative fever. Pneumonia is a major lung complication associated with increased morbidity and mortality, length of hospital stay, and likelihood of hospital readmissions.[2] In conjunction with breathing exercises and early mobility, incentive spirometry use is therefore beneficial for patients recovering from pneumonia or rib damage to help minimize the chance of fluid build-up in the lungs. Because of its role in pulmonary rehabilitation and inspiratory muscle training, this device may theoretically benefit patients with COVID-19.[3] It may be used as well by wind instrument players who want to improve their air flow.
Indications
Incentive spirometer is indicated for the following reasons:[4]
- Following abdominal or thoracic surgery to reduce risks of pulmonary complications including atelectasis and pneumonia
- Patients with prolonged immobilization and bed rest
- Chronic Obstructive Pulmonary Disease
- COVID-19[5][6]
- Cystic Fibrosis
- Sickle Cell Anemia
- Mild to moderate asthma
- Traumatic rib fractures with subsequent complications of hemothorax and flail chest[7]
Contraindications
While there are no absolute contraindications for spirometry use, inspiratory muscle training can worsen some existing medical conditions, including the following:[4]
- Recent or current respiratory tract infection
- Hemoptysis of unknown origin
- Pneumothorax
- Emphysema, due to increased barotrauma[8]
- Thoracic, abdominal, or cerebral aneurysms
- Recent thoracic, abdominal, or eye surgery
- Nausea, vomiting, or acute illness
- Confusion or dementia
- Undiagnosed hypertension
Usage
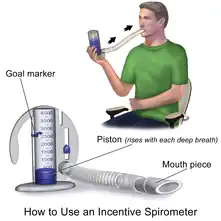
Patient starts in a seated upright position. Patient exhales completely before using device. The patient then places the mouthpiece into their mouth and seals their lips tightly around it. The patient breathes in from the device as slowly and as deeply as possible, then holds that breath in for 2–6 seconds. This provides back pressure that pops open alveoli. It has the same effect as that which occurs during yawning. An indicator piston driven by the patient's breathing provides a gauge of how well the patient's lungs (or lung if singular) are functioning, by indicating sustained inhalation vacuum. While the patient is holding their breath, the indicator piston will slowly return to the bottom of the column. Patient then removes the mouthpiece from their mouth and exhales normally. Coughing can be expected to clear the airway and lungs of mucus. Patients are encouraged to rest if they begin feeling dizzy.[1]
Generally, patients are encouraged to do many repetitions a day while measuring progress by way of advancing the movable gauge along the central column of the device as they improve.
Alternative approach for children
Traditional incentive spirometers can be more challenging for children due to compliance and submaximal effort.[9] Age-appropriate devices including whistles, pinwheels, and bubble wands should be considered.[10] These toys and activities reinforce proper breathing mechanics by stimulating deep inhalation and prolonged exhalation.
Additional images

See also
- Spirometer, a device for measuring lung capacity
- Respiratory system
References
- 1 2 "How to use an incentive spirometer". The Cleveland Clinic Foundation. Retrieved 2013-11-03.
- ↑ Chughtai, Morad; Gwam, Chukwuweike U.; Mohamed, Nequesha; Khlopas, Anton; Newman, Jared M.; Khan, Rafay; Nadhim, Ali; Shaffiy, Shervin; Mont, Michael A. (June 2017). "The Epidemiology and Risk Factors for Postoperative Pneumonia". Journal of Clinical Medicine Research. 9 (6): 466–475. doi:10.14740/jocmr3002w. ISSN 1918-3003. PMC 5412519. PMID 28496546.
- ↑ Seyller, Hannah; Gottlieb, Michael; Colla, Joseph (2021-10-01). "A breath of fresh air: The role of incentive spirometry in the treatment of COVID-19". The American Journal of Emergency Medicine. 48: 369. doi:10.1016/j.ajem.2021.01.084. ISSN 0735-6757. PMC 8500986. PMID 33558097.
- 1 2 Franklin, Emily; Anjum, Fatima (2021), "Incentive Spirometer and Inspiratory Muscle Training", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 34283480, retrieved 2021-11-12
- ↑ Bernal-Utrera, Carlos; Anarte-Lazo, Ernesto; Gonzalez-Gerez, Juan Jose; De-La-Barrera-Aranda, Elena; Saavedra-Hernandez, Manuel; Rodriguez-Blanco, Cleofas (February 2021). "Could Physical Therapy Interventions Be Adopted in the Management of Critically Ill Patients with COVID-19? A Scoping Review". International Journal of Environmental Research and Public Health. 18 (4): 1627. doi:10.3390/ijerph18041627. ISSN 1661-7827. PMC 7915254. PMID 33567748.
- ↑ Shan, Mia X.; Tran, Yen M.; Vu, Kim T.; Eapen, Blessen C. (2020-08-18). "Postacute inpatient rehabilitation for COVID-19". BMJ Case Reports. 13 (8): e237406. doi:10.1136/bcr-2020-237406. ISSN 1757-790X. PMC 7437681. PMID 32816941.
- ↑ Sum, Shao-Kai; Peng, Ya-Chuan; Yin, Shun-Ying; Huang, Pin-Fu; Wang, Yao-Chang; Chen, Tzu-Ping; Tung, Heng-Hsin; Yeh, Chi-Hsiao (2019-12-30). "Using an incentive spirometer reduces pulmonary complications in patients with traumatic rib fractures: a randomized controlled trial". Trials. 20 (1): 797. doi:10.1186/s13063-019-3943-x. ISSN 1745-6215. PMC 6937666. PMID 31888765.
- ↑ Kenny, Jon-Emile S.; Kuschner, Ware G. (July 2013). "Pneumothorax caused by aggressive use of an incentive spirometer in a patient with emphysema". Respiratory Care. 58 (7): e77–79. doi:10.4187/respcare.02130. ISSN 1943-3654. PMID 23232741. S2CID 74429.
- ↑ Gupta, Anish; Mishra, Priyanka; Gupta, Bhavna; Kakkar, Kamna (April 2021). "A kid-friendly approach to Incentive Spirometry". Annals of Cardiac Anaesthesia. 24 (2): 238–240. doi:10.4103/aca.ACA_188_19. ISSN 0974-5181. PMC 8253039. PMID 33884984.
- ↑ Schivinski, Camila Isabel Santos; Manna, Bruna Cardoso; Belém, Fabíula Joanita da Mata; Castilho, Tayná (2020-03-09). "Therapeutic Blowing Toys: Does the Overlap of Ventilatory Stimuli Alter the Respiratory Mechanics of Healthy Schoolchildren?". Revista Paulista de Pediatria. 38: e2018259. doi:10.1590/1984-0462/2020/38/2018259. ISSN 0103-0582. PMC 7063596. PMID 32159645.
External links
| Wikimedia Commons has media related to Incentive spirometer. |