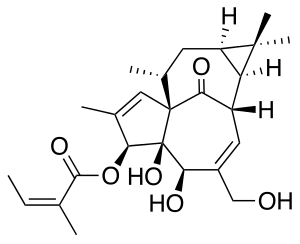
Ingenol mebutate
 | |
| Names | |
|---|---|
| Trade names | Picato |
| Other names | PEP005, ingenol-3-angelate |
IUPAC name
| |
| Clinical data | |
| Drug class | Macrocyclic diterpene ester[1] |
| Main uses | Actinic keratosis[1] |
| Side effects | Skin cancer, redness, skin peeling, blistering, itchiness[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Topical (gel) |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613008 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | Below detection level |
| Chemical and physical data | |
| Formula | C25H34O6 |
| Molar mass | 430.541 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ingenol mebutate, sold under the brand name Picato, is a medication used to treat actinic keratosis.[1] It is applied to the skin once per day for 2 to 3 days.[1] It appears to work the same as fluorouracil or imiquimod.[1]
Common side effects include redness, skin peeling, blistering, and itchiness.[1] Other side effects may include skin cancer in an extra 4% of people, allergic reactions, and shingles.[2][1] Safety in pregnancy is unclear.[1] It is a macrocyclic diterpene ester.[1]
Ingenol mebutate was approved for medical use in the United States in 2012.[1] While it was approved in Europe in 2012, it was subsequently withdrawn due to concerns regarding side effects.[3][2] In the United States three doses costs about 1,100 USD as of 2021.[4] It from the sap of the milkweed plant.[1]
Medical use
Ingenol mebutate gel applied topically, for 2 to the trunk or 3 days to the face or scalp, is effective for field treatment of actinic keratoses.[5][6]
A twelve-month follow-up study was performed on actinic keratosis patients who had been treated with ingenol mebutate, 108 of which had been treated for face or scalp and 71 for trunk or extremities and the study found that of those treated for the face or scalp 46.1% had sustained clearance, and of those treated for the trunk 44.0% had sustained clearance for the period of the study.[7]
.jpg.webp) Day 0 on actinic keratoses
Day 0 on actinic keratoses.jpg.webp) Day 2
Day 2.jpg.webp) Day 6
Day 6
Dosage
Two different strengths of the gel have been approved for use on either the face and scalp (0.015%) or the trunk and extremities (0.05%), respectively.[8]
Side effects
Irritation of the application site is very common. The various types of irritation include redness, scaling, crusting, pain, severe itching, and sometimes infection. Additional possible side effects include eye irritation, such as periorbital edema (3% of patients in studies), headaches (2%) and nasopharyngitis (running nose, 2%).[9]
Allergic reactions, shingles, changes in pigmentation at application site, chemical conjunctivitis, and corneal burns may also occur.[10][11]
The European Medicines Agency recommended suspending the marketing authorisation of ingenol mebutate due to concerns regarding increased incidence of skin cancer in patients treated with topical ingenol mebutate compared to vehicle or imiquimod. Physicians were advised to refrain from prescribing ingenol and to use different treatment options.[12] Subsequently, the marketing authorization holder decided to request withdrawal of the manufacturing authorization for commercial reasons. The withdrawal was granted and therefore, ingenol mebutate is no longer registered in the EU.[13]
Skin cancer
Health Canada assessed twelve studies published in scientific and medical literature in order to determine the link between the use of ingenol mebutate and skin cancer. Health Canada's review found that six of the twelve studies had evidence of skin cancer with the use of ingenol mebutate. The European Medicines Agency (EMA) has also reviewed this safety issue. In April 2020, it concluded that ingenol mebutate may increase the risk of skin cancer and that its risks outweigh its benefits. On February 11, 2020, the manufacturer voluntarily withdrew the product from the European Union market.[14]
Interactions
As ingenol mebutate is not effectively absorbed through the skin and into the bloodstream, interactions with oral drugs are unlikely.[6][15]
Chemistry

The substance is an ester of the diterpene ingenol and angelic acid. A 3-step semisynthesis of ingenol mebutate starting from ingenol was described by a chemical research group in Denmark in 2012.[16] A 14-step synthesis of (+)-ingenol from (+)-3-carene, which is a relatively inexpensive constituent of turpentine, was published in July 2013.[17]
Mechanism of action
The mechanism by which ingenol mebutate causes cell death is still not fully understood. One study on squamous cell carcinoma, the precursor of which is actinic keratosis, cultures found that the PKC/MEK/ERK signaling pathway is involved in causing cell death after treatment with ingenol mebutate. In addition, the interleukin decoy receptors IL1R2 and IL13RA2 were induced, resulting in a reduction in the long-term viability of the cells, which could help prevent recurrence.[18]
Research
HIV
Ingenol mebutate has also been found to be useful for reactivating latent HIV virus in cells taken from individuals who have tested negative for signs of the disease following extended courses of anti-retroviral drugs, raising the possibility that this drug may be used to expose the last traces of virus, and thus potentially provide a permanent cure for HIV infection. Research is ongoing to determine whether the effects observed in vitro are also seen in animal models, with a view to eventual human trials for this application.[19]
Tattoo removal
A study on hairless mice found that 0.1% ingenol mebutate gel was able to remove two-week-old tattoos consistently. It was observed that the microspheres within the skin containing the dye would exude into the scab intact and slough off as the skin healed about 20 days after treatment began. This mechanism appears to be independent of ink color, unlike laser tattoo removal, which is less effective for certain colors. Human trials have not yet been conducted.[20]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "Ingenol Mebutate Monograph for Professionals". Drugs.com. Archived from the original on 5 March 2021. Retrieved 26 November 2021.
- 1 2 "Risks of Picato for actinic keratosis outweigh benefits". Archived from the original on 5 January 2021. Retrieved 26 November 2021.
- ↑ "Picato". Archived from the original on 28 August 2021. Retrieved 26 November 2021.
- ↑ "Picato Prices and Picato Coupons - GoodRx". GoodRx. Archived from the original on 1 October 2016. Retrieved 26 November 2021.
- ↑ Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B (2012). "Ingenol Mebutate Gel for Actinic Keratosis". N Engl J Med. 366 (11): 1010–1019. doi:10.1056/NEJMoa1111170. PMID 22417254.
- 1 2 Picato FDA Professional Drug Information
- ↑ Lebwohl M, Shumack S, Gold L (2013). "Long-term Follow-up Study of Ingenol Mebutate Gel for the Treatment of Actinic Keratoses". JAMA Dermatology. 149 (6): 666–670. doi:10.1001/jamadermatol.2013.2766. PMID 23553119. Archived from the original on 2020-10-26. Retrieved 2020-12-27.
- ↑ "Picato® Gel label at Drugs@FDA" (PDF). Archived (PDF) from the original on 2021-01-21. Retrieved 2020-12-27.
- ↑ Drugs.com: Picato Side Effects in Detail Archived 2019-08-03 at the Wayback Machine
- ↑ "Picato (ingenol mebutate) Gel: Drug Safety Communication - FDA Warns of Severe Adverse Events, Requires Label Changes". 2015-08-21. Archived from the original on 2015-08-24. Retrieved 24 August 2015.
- ↑ "Picato (Ingenol Mebutate): Side Effects, Interactions, Warning, Dosage & Uses". RxList. Archived from the original on 2018-12-06. Retrieved 2018-12-06.
- ↑ "Archive copy". Archived from the original on 2021-01-06. Retrieved 2020-12-27.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Archive copy" (PDF). Archived (PDF) from the original on 2021-08-29. Retrieved 2020-12-27.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Government of Canada, Health Canada (2014-10-23). "Search Page - Drug and Health Product Register". hpr-rps.hres.ca. Archived from the original on 2020-10-30. Retrieved 2020-10-27.
- ↑ Haberfeld, H, ed. (2013). Austria-Codex (in Deutsch). Vienna: Österreichischer Apothekerverlag.
- ↑ Liang, X.; Grue-Sørensen, G.; Petersen, A. K.; Högberg, T. (2012), "Semisynthesis of Ingenol 3-Angelate (PEP005): Efficient Stereoconservative Angeloylation of Alcohols", SYNLETT (23): 2647–2652. https://www.thieme-connect.com/products/ejournals/pdf/10.1055/s-0032-1317415.pdf Archived 2021-08-29 at the Wayback Machine
- ↑ Jorgensen, L.; McKerrall, S. J.; Kuttruff, C. A.; Ungeheuer, F.; Felding, J.; Baran, P. S. (2013). "14-Step Synthesis of (+)-Ingenol from (+)-3-Carene". Science. 341 (6148): 878–882. Bibcode:2013Sci...341..878J. doi:10.1126/science.1241606. PMID 23907534. S2CID 26998997.
- ↑ Freiberger S (2015). "Ingenol Mebutate Signals via PKC/MEK/ERK in Keratinocytes and Induces Interleukin Decoy Receptors IL1R2 and IL13RA2". Molecular Cancer Therapeutics. 14 (9): 2132–2142. doi:10.1158/1535-7163.mct-15-0023-t. PMID 26116359. Archived from the original on 2020-08-05. Retrieved 2020-12-27.
- ↑ Jiang G, Mendes EA, Kaiser P, Wong DP, Tang Y, Cai I, et al. (2015). "Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation". PLOS Pathogens. 11 (7): e1005066. doi:10.1371/journal.ppat.1005066. PMC 4520526. PMID 26225771.
- ↑ Cozzi S (2017). "Tattoo Removal with Ingenol Mebutate". Clinical, Cosmetic and Investigational Dermatology. 10: 205–210. doi:10.2147/ccid.s135716. PMC 5448692. PMID 28579816. Archived from the original on 2021-08-28. Retrieved 2020-12-27.
External links
| Identifiers: |
|---|
- "Ingenol mebutate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-08-28. Retrieved 2020-12-27.