Neuroscience of sex differences
| Part of a series on |
| Sex differences in humans |
|---|
| Biology |
| Medicine and Health |
|
| Neuroscience and Psychology |
|
| Sociology |
|
The neuroscience of sex differences is the study of characteristics that separate the male and female brain. Psychological sex differences are thought by some to reflect the interaction of genes, hormones, and social learning on brain development throughout the lifespan.
A 2021 meta-synthesis of existing literature found that sex accounted for 1% of the brain's structure or laterality, finding large group-level differences only in total brain volume.[1] A review from 2006 and a meta-analysis from 2014 found that some evidence from brain morphology and function studies indicates that male and female brains cannot always be assumed to be identical from either a structural or functional perspective, and some brain structures are sexually dimorphic.[2][3]
History
The ideas of differences between the male and female brain have circulated since the time of Ancient Greek philosophers around 850 BC. In 1854, German anatomist Emil Huschke discovered a size difference in the frontal lobe, where male frontal lobes are 1% larger than those of females.[4] As the 19th century progressed, scientists began researching sexual dimorphisms in the brain significantly more.[5] Until recent decades, scientists knew of several structural sexual dimorphisms of the brain, but they did not think that sex had any impact on how the human brain performs daily tasks. Through molecular, animal, and neuroimaging studies, a great deal of information regarding the differences between male and female brains and how much they differ in regards to both structure and function has been uncovered.[6]
Evolutionary explanations
Sexual selection
Females show enhanced information recall compared to males. This may be due to the fact that females have a more intricate evaluation of risk–scenario contemplation, based on a prefrontal cortical control of the amygdala. For example, the ability to recall information better than males most likely originated from sexual selective pressures on females during competition with other females in mate selection. Recognition of social cues was an advantageous characteristic, because it ultimately maximized offspring and was therefore selected for during evolution.[2]
Oxytocin is a hormone that induces contraction of the uterus and lactation in mammals and is also a characteristic hormone of nursing mothers. Studies have found that oxytocin improves spatial memory. Through activation of the MAP kinase pathway, oxytocin plays a role in the enhancement of long-term synaptic plasticity, which is a change in strength between two neurons over a synapse that lasts for minutes or longer, and long-term memory. This hormone may have helped mothers remember the location of distant food sources so they could better nurture their offspring.[2]
Male and female brain anatomy
A 2021 meta-synthesis of existing literature found that sex accounted for 1% of the brain's structure or laterality, finding large group-level differences only in total brain volume.[7]
Many similarities and differences in structure, neurotransmitters, and function have been identified,[3][8] but some academics, such as Cordelia Fine[9] and Anelis Kaiser, Sven Haller, Sigrid Schmitz, and Cordula Nitsch[10] dispute the existence of significant sex differences in the brain, arguing that innate differences in the neurobiology of women and men have not been conclusively identified due to factors such as neurosexism, methodological flaws and publication bias.[9][10]
Males and females differ in some aspects of their brains, notably the overall difference in size, with men having larger brains on average (between 8% and 13% larger),[3] but a relationship between brain volume or density and brain function is not established.[11] Additionally, there are differences in activation patterns that suggest anatomical or developmental differences.
Volume
Structurally, adult male brains are on average 11–12% heavier and 10% bigger than female brains.[12] Though statistically there are sex differences in white matter and gray matter percentage, this ratio is directly related to brain size, and some[13] argue these sex differences in gray and white matter percentage are caused by the average size difference between men and women. Others argue that these differences partly remain after controlling for brain volume.[8][14]
Researchers also found greater cortical thickness and cortical complexity in females and greater female cortical surface area after adjusting for brain volumes.[14] Given that cortical complexity and cortical features have had some evidence of positive correlation with intelligence, researchers postulated that these differences might have evolved for females to compensate for smaller brain size and equalize overall cognitive abilities with males.[14]
According to the neuroscience journal review series Progress in Brain Research, it has been found that males have larger and longer planum temporale and Sylvian fissure while females have significantly larger proportionate volumes to total brain volume in the superior temporal cortex, Broca's area, the hippocampus and the caudate.[14] The midsagittal and fiber numbers in the anterior commissure that connect the temporal poles and mass intermedia that connects the thalami is also larger in women.[14]
Lateralization
Lateralization may differ between the sexes, with men often being said to have a more lateralized brain. This is often attributed to differences in "left-" and "right-" brained abilities. One factor that contributes support to the idea that there is a sex difference in brain lateralization is that men are more likely to be left-handed. However, it is unclear whether this is due to a difference in lateralization.[15]
A 2014 meta-analysis of grey matter in the brain found sexually dimorphic areas of the brain in both volume and density. When synthesized, these differences show that volume increases for males tend to be on the left side of systems, while females generally see greater volume in the right hemisphere.[3] On the other hand, a previous 2008 meta-analysis found that the difference between male and female brain lateralization was not significant.[15]
Amygdala
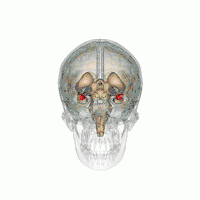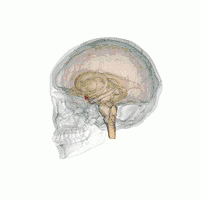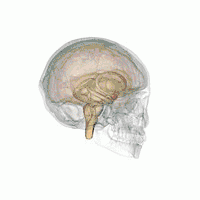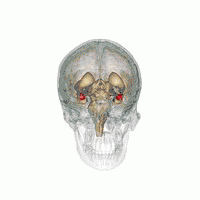
There are behavioral differences between males and females that may suggest a difference in amygdala size or function. A 2017 review of amygdala volume studies found that there was a raw size difference, with males having a 10% larger amygdala, however, because male brains are larger, this finding was found to be misleading. After normalizing for brain size, there was no significant difference in size of the amygdala across sex.[16]
In terms of activation, there is no difference in amygdala activation across sex. Differences in behavioral tests may be due to potential anatomical and physiological differences in the amygdala across sexes rather than activation differences.[17]
Emotional expression, understanding, and behavior appears to vary between males and females. A 2012 review concluded that males and females have differences in the processing of emotions. Males tend to have stronger reactions to threatening stimuli and that males react with more physical violence.[18]
Hippocampus
Hippocampus atrophy is associated with a variety of psychiatric disorders that have higher prevalence in females. Additionally, there are differences in memory skills between males and females which may suggest a difference in the hippocampal volume (HCV). A 2016 meta-analysis of volume differences found a higher HCV in males without correcting for total brain size. However, after adjusting for individual differences and total brain volume, they found no significant sex difference, despite the expectation that women may have larger hippocampus volume.[19]
Grey matter
A 2014 meta-analysis found (where differences were measured) some differences in grey matter levels between the sexes.
The findings included females having more grey matter volume in the right frontal pole, inferior and middle frontal gyrus, pars triangularis, planum temporale/parietal operculum, anterior cingulate gyrus, insular cortex, and Heschl's gyrus; both thalami and precuneus; the left parahippocampal gyrus and lateral occipital cortex (superior division).[3] Larger volumes in females were most pronounced in areas in the right hemisphere related to language in addition to several limbic structures such as the right insular cortex and anterior cingulate gyrus.[3]
Males had more grey matter volume in both amygdalae, hippocampi, anterior parahippocampal gyri, posterior cingulate gyri, precuneus, putamen and temporal poles, areas in the left posterior and anterior cingulate gyri, and areas in the cerebellum bilateral VIIb, VIIIa and Crus I lobes, left VI and right Crus II lobes.[3]
In terms of density, there were also differences between the sexes. Males tended to have a denser left amygdala, hippocampus, insula, pallidum, putamen, claustrum, and areas of the right VI lobule of the cerebellum, among other areas.[3] Females tended to have denser left frontal pole.[3]
The significance of these differences lies both in the lateralization (males having more volume in the left hemisphere and females having more volume in the right hemisphere) and the possible uses of these findings to explore differences in neurological and psychiatric conditions.
Transgender studies on brain anatomy
Early postmortem studies of transgender neurological differentiation were focused on the hypothalamic and amygdala regions of the brain. Using magnetic resonance imaging (MRI), some trans women were found to have female-typical putamina that were larger in size than those of cisgender males.[20] Some trans women have also shown a female-typical central part of the bed nucleus of the stria terminalis (BSTc) and interstitial nucleus of the anterior hypothalamus number 3 (INAH-3), looking at the number of neurons found within each.[21]
Brain networks
Both males and females have consistent active working memory networks composed of both middle frontal gyri, the left cingulate gyrus, the right precuneus, the left inferior and superior parietal lobes, the right claustrum, and the left middle temporal gyrus.[22] Although the same brain networks are used for working memory, specific regions are sex-specific. Sex differences were evident in other networks, as women also tend to have higher activity in the prefrontal and limbic regions, such as the anterior cingulate, bilateral amygdala, and right hippocampus, while men tend to have a distributed network spread out among the cerebellum, portions of the superior parietal lobe, the left insula, and bilateral thalamus.[22]
A 2017 review from the perspective of large-scale brain networks hypothesized that women's higher susceptibility to stress-prone diseases such as posttraumatic stress disorder and major depressive disorder, in which the salience network is theorized to be overactive and to interfere with the executive control network, may be due in part, along with societal exposure to stressors and the coping strategies that are available to women, to underlying sex-based brain differences.[23]
Neurochemical differences
Hormones
Gonadal hormones, or sex hormones, include androgens (such as testosterone) and estrogens (such as estradiol), which are steroid hormones synthesized primarily in the testes and ovaries, respectively. Sex hormone production is regulated by the gonadotropic hormones luteinizing hormone (LH) and follicle-stimulating hormone (FSH), whose release from the anterior pituitary is stimulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus.[24]
Steroid hormones have several effects on brain development as well as maintenance of homeostasis throughout adulthood. Estrogen receptors have been found in the hypothalamus, pituitary gland, hippocampus, and frontal cortex, indicating the estrogen plays a role in brain development. Gonadal hormone receptors have also been found in the basal fore-brain nuclei.[25]
Estrogen and the female brain
Estradiol influences cognitive function, specifically by enhancing learning and memory in a dose-sensitive manner. Too much estrogen can have negative effects by weakening performance of learned tasks as well as hindering performance of memory tasks; this can result in females exhibiting poorer performance of such tasks when compared to males.[26]
Ovariectomies, surgeries inducing menopause, or natural menopause cause fluctuating and decreased estrogen levels in women. This in turn can "attenuate the effects" of endogenous opioid peptides. Opioid peptides are known to play a role in emotion and motivation. The content of β-endorphin (β-EP), an endogenous opioid peptide, has been found to decrease (in varying amounts/brain region) post ovariectomy in female rats within the hypothalamus, hippocampus, and pituitary gland. Such a change in β-EP levels could be the cause of mood swings, behavioral disturbances, and hot flashes in post menopausal women.[25]
Progesterone and the male and female brain
Progesterone is a steroid hormone synthesized in both male and female brains. It contains characteristics found in the chemical nucleus of both estrogen and androgen hormones.[27] As a female sex hormone, progesterone is more significant in females than in males. During the menstrual cycle, progesterone increases just after the ovulatory phase to inhibit luteinizing hormones, such as oxytocin absorption.[28] In men, increased progesterone has been linked to adolescents with suicidal ideation.[29]
Testosterone and the male brain
The gonadal hormone testosterone is an androgenic, or masculinizing, hormone that is synthesized in both the male testes and female ovaries,[30] at a rate of about 14,000 μg/day and 600 μg/day, respectively.[24] Testosterone exerts organizational effects on the developing brain, many of which are mediated through estrogen receptors following its conversion to estrogen by the enzyme aromatase within the brain.[24]
See also
- Biology and sexual orientation
- Etiology of transsexualism
- Neuroplasticity
- Neuroscience and sexual orientation
- Neuroscience
- Puberty § Neurohormonal process
- Rett syndrome
- Sex differences in human psychology
- Sexually dimorphic nucleus
- The NeuroGenderings Network
References
- ↑ Eliot, Lise; Ahmed, Adnan; Khan, Hiba; Patel, Julie (2021-06-01). "Dump the "dimorphism": Comprehensive synthesis of human brain studies reveals few male-female differences beyond size". Neuroscience & Biobehavioral Reviews. 125: 667–697. doi:10.1016/j.neubiorev.2021.02.026. ISSN 0149-7634. PMID 33621637.
- 1 2 3 Cahill L (June 2006). "Why sex matters for neuroscience". Nature Reviews. Neuroscience. 7 (6): 477–84. doi:10.1038/nrn1909. PMID 16688123. S2CID 10847255.
- 1 2 3 4 5 6 7 8 9 Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J (February 2014). "A meta-analysis of sex differences in human brain structure". Neuroscience and Biobehavioral Reviews (Meta-analysis). 39: 34–50. doi:10.1016/j.neubiorev.2013.12.004. PMC 3969295. PMID 24374381.
- ↑ Swaab DF, Hofman MA (1984). Sexual differentiation of the human brain. A historical perspective. Progress in Brain Research. Vol. 61. pp. 361–74. doi:10.1016/S0079-6123(08)64447-7. ISBN 9780444805324. PMID 6396708.
- ↑ Hofman MA, Swaab DF (1991). "Sexual dimorphism of the human brain: myth and reality" (PDF). Experimental and Clinical Endocrinology. 98 (2): 161–70. doi:10.1055/s-0029-1211113. PMID 1778230.
- ↑ McCarthy MM (February 2016). "Multifaceted origins of sex differences in the brain". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 371 (1688): 20150106. doi:10.1098/rstb.2015.0106. PMC 4785894. PMID 26833829.
- ↑ Eliot, Lise; Ahmed, Adnan; Khan, Hiba; Patel, Julie (2021-06-01). "Dump the "dimorphism": Comprehensive synthesis of human brain studies reveals few male-female differences beyond size". Neuroscience & Biobehavioral Reviews. 125: 667–697. doi:10.1016/j.neubiorev.2021.02.026. ISSN 0149-7634. PMID 33621637.
- 1 2 Cosgrove, Kelly P.; Mazure, Carolyn M.; Staley, Julie K. (2007). "Evolving Knowledge of Sex Differences in Brain Structure, Function, and Chemistry". Biological Psychiatry (Review). 62 (8): 847–55. doi:10.1016/j.biopsych.2007.03.001. PMC 2711771. PMID 17544382.
- 1 2 Fine, Cordelia (2010). Delusions of Gender: How Our Minds, Society, and Neurosexism Create Difference. W. W. Norton. ISBN 978-0393068382.
- 1 2 Kaiser, Anelis; Haller, Sven; Schmitz, Sigrid; Nitsch, Cordula (2009). "On sex/gender related similarities and differences in fMRI language research". Brain Research Reviews (Review). 61 (2): 49–59. doi:10.1016/j.brainresrev.2009.03.005. PMID 19406148. S2CID 17275248.
- ↑ Nisbett, Richard E.; Aronson, Joshua; Blair, Clancy; Dickens, William; Flynn, James; Halpern, Diane F.; Turkheimer, Eric (February 2012). "Intelligence: New findings and theoretical developments". American Psychologist (Review). 67 (2): 130–159. doi:10.1037/a0026699. PMID 22233090.
- ↑ O'Brien, Jodi (2009). Encyclopedia of Gender and Society. Los Angeles: SAGE. p. 343. ISBN 978-1-4129-0916-7.
- ↑ Reiss, Allan L.; Abrams, Michael T.; Singer, Harvey S.; Ross, Judith L.; Denckla, Martha B. (October 1996). Brain development, gender and IQ in children: A volumetric imaging study. Volume 119, Issue 5: Brain. pp. 1763–1774.
{{cite book}}: CS1 maint: location (link) - 1 2 3 4 5 Savic, I. (2010). Sex Differences in the Human Brain, their Underpinnings and Implications. Progress in Brain Research (Journal review series). ISSN. Elsevier Science. ISBN 978-0-444-53631-0. Retrieved 2021-12-26.
- 1 2 Sommer IE, Aleman A, Somers M, Boks MP, Kahn RS (April 2008). "Sex differences in handedness, asymmetry of the planum temporale and functional language lateralization". Brain Research. 1206: 76–88. doi:10.1016/j.brainres.2008.01.003. PMID 18359009. S2CID 7371496.
- ↑ Marwha D, Halari M, Eliot L (February 2017). "Meta-analysis reveals a lack of sexual dimorphism in human amygdala volume". NeuroImage. 147: 282–294. doi:10.1016/j.neuroimage.2016.12.021. PMID 27956206. S2CID 3479632.
- ↑ Sergerie K, Chochol C, Armony JL (2008). "The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies". Neuroscience and Biobehavioral Reviews. 32 (4): 811–30. doi:10.1016/j.neubiorev.2007.12.002. PMID 18316124. S2CID 10980762.
- ↑ Kret ME, De Gelder B (June 2012). "A review on sex differences in processing emotional signals" (PDF). Neuropsychologia. 50 (7): 1211–21. doi:10.1016/j.neuropsychologia.2011.12.022. PMID 22245006. S2CID 11695245.
- ↑ Tan A, Ma W, Vira A, Marwha D, Eliot L (January 2016). "The human hippocampus is not sexually-dimorphic: Meta-analysis of structural MRI volumes". NeuroImage. 124 (Pt A): 350–366. doi:10.1016/j.neuroimage.2015.08.050. PMID 26334947. S2CID 26316768.
- ↑ Saleem F, Rizvi SW (December 2017). "Transgender Associations and Possible Etiology: A Literature Review". Cureus. 9 (12): e1984. doi:10.7759/cureus.1984. PMC 5825045. PMID 29503778.
- ↑ Guillamon A, Junque C, Gómez-Gil E (October 2016). "A Review of the Status of Brain Structure Research in Transsexualism". Archives of Sexual Behavior. 45 (7): 1615–48. doi:10.1007/s10508-016-0768-5. PMC 4987404. PMID 27255307.
- 1 2 Hill AC, Laird AR, Robinson JL (October 2014). "Gender differences in working memory networks: a BrainMap meta-analysis" (PDF). Biological Psychology. 102: 18–29. doi:10.1016/j.biopsycho.2014.06.008. PMC 4157091. PMID 25042764.
- ↑ Homberg JR, Kozicz T, Fernandez G (April 2017). "Large-scale network balances in the transition from adaptive to maladaptive stress responses". Current Opinion in Behavioral Sciences. 14: 27–32. doi:10.1016/j.cobeha.2016.11.003. S2CID 53161342.
- 1 2 3 Molina PE (2018). Endocrine physiology (5th ed.). New York: McGraw-Hill Education. ISBN 9781260019360. OCLC 1026417940.
- 1 2 Genazzani AR, Pluchino N, Luisi S, Luisi M (2007). "Estrogen, cognition and female ageing". Human Reproduction Update. 13 (2): 175–87. doi:10.1093/humupd/dml042. PMID 17135285.
- ↑ Korol DL (November 2004). "Role of estrogen in balancing contributions from multiple memory systems". Neurobiology of Learning and Memory. 82 (3): 309–23. doi:10.1016/j.nlm.2004.07.006. PMID 15464412. S2CID 19893375.
- ↑ Funk and Wagnalls (2018). Progesterone. World Almanac Education Group.
- ↑ Ulshöfer, Gotlind; Karafyllis, Nicole (2008). Sexualized Brains: Scientific Modeling of Emotional Intelligence from a Cultural Perspective. Cambridge, Massachusetts: The MIT Press. p. 213.
- ↑ Lester, David; Gunn, John F. III; Quinnett, Paul, eds. (2014). Suicide in Men: How Men Differ from Women in Expressing their Distress. Springfield, Illinois: Charles C Thomas. p. 61. ISBN 978-0-398-08794-4.
- ↑ Hadley ME, Levine JE (2007). Endocrinology. Levine, Jon E. (6th, New ed.). Upper Saddle River, NJ: Pearson Prentice Hall. ISBN 978-0131876064. OCLC 70929277.
Further reading
- Rippon, Gina (28 Feb 2019). The gendered brain: The new neuroscience that shatters the myth of the female brain. Bodley Head. ISBN 978-1847924759.