Rett syndrome
| Rett syndrome | |
|---|---|
| Other names: Cerebroatrophic hyperammonemia;[1] autism, dementia, ataxia, and loss of purposeful hand use syndrome[2] | |
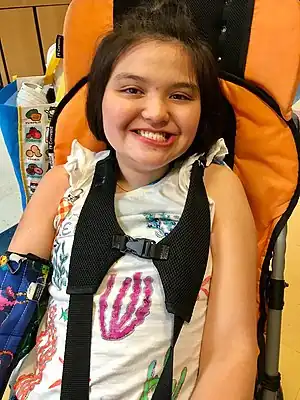 | |
| A girl with Rett Syndrome smiling at the camera | |
| Specialty | Psychiatry, pediatrics |
| Symptoms | Impairments in language and coordination, and repetitive movements, slower growth, smaller head[3] |
| Complications | Seizures, scoliosis, sleeping problems[3] |
| Usual onset | After 6–18 months of age[3] |
| Duration | Lifelong[4] |
| Causes | Mutation in the MECP2 gene[3] |
| Diagnostic method | Based on symptoms, genetic testing[4] |
| Differential diagnosis | Angelman syndrome, autism, cerebral palsy, various neurodegenerative disorders[5] |
| Treatment | Special education, physiotherapy, braces[4] |
| Medication | Anticonvulsants[4] |
| Prognosis | Life expectancy for many is middle age.[4] |
| Frequency | 1 in 8,500 females[3] |
Rett syndrome (RTT) is a genetic disorder that typically becomes apparent after 6–18 months of age in females.[3] Symptoms include impairments in language and coordination and repetitive movements.[3] Those affected often have slower growth, difficulty walking, and a smaller head size.[3][4] Complications of Rett syndrome can include seizures, scoliosis, and sleeping problems.[3] The severity of the condition is variable, however.[4]
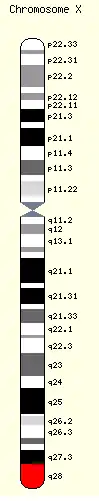
Rett syndrome is due to a genetic mutation in the MECP2 gene,[3] on the X chromosome.[4] It almost always occurs as a new mutation, with less than one percent of cases being inherited from a person's parents.[3][4] It occurs almost exclusively in girls;[3] boys who have a similar mutation typically die shortly after birth.[4] Diagnosis is based on the symptoms and can be confirmed with genetic testing.[4]
There is no known cure for Rett syndrome.[4] Treatment is directed at improving symptoms.[4] Anticonvulsants may be used to help with seizures.[4] Special education, physiotherapy, and braces may also be useful.[4] Many of those with the condition live into middle age.[4]
The condition affects about 1 in 8,500 females.[3] It was first described by Austrian pediatrician Andreas Rett in 1966.[4][6] As his writings were in German, they did not become widely known in the English-speaking world.[7] Swedish pediatrician Bengt Hagberg published an English article in 1983 and named the condition after Rett.[7] In 1999, Lebanese-American physician Huda Zoghbi discovered the mutation that causes the condition.[7][8]
Signs and symptoms
Stage I
Stage I, called early-onset, typically begins between 6 and 18 months of age.[4] This stage is often overlooked because symptoms of the disorder may be somewhat vague, and parents and doctors may not notice the subtle slowing of development at first.[4] The infant may begin to show less eye contact and have reduced interest in toys. There may be delays in gross motor skills such as sitting or crawling.[4] Hand-wringing and decreasing head growth may occur, but not enough to draw attention. This stage usually lasts for a few months but can continue for more than a year.[4]
Stage II
Stage II, or the rapid destructive stage, usually begins between ages 1 and 4 and may last for weeks or months.[4] Its onset may be rapid or gradual as the child loses purposeful hand skills and spoken language.[4] Characteristic hand movements such as wringing, washing, clapping, or tapping, as well as repeatedly moving the hands to the mouth often begin during this stage.[4] The child may hold the hands clasped behind the back or held at the sides, with random touching, grasping, and releasing.[4] The movements continue while the child is awake but disappear during sleep.[4] Breathing irregularities such as episodes of apnea and hyperventilation may occur, although breathing usually improves during sleep.[4] Some girls also display autistic-like symptoms such as loss of social interaction and communication.[4] Walking may be unsteady and initiating motor movements can be difficult. Slowed head growth is usually noticed during this stage.[4]
Stage III
Stage III, or the plateau or pseudo-stationary stage, usually begins between ages 2 and 10 and can last for years.[4] Apraxia, motor problems, and seizures are prominent during this stage.[4] However, there may be improvement in behavior, with less irritability, crying, and autistic-like features.[4] In stage III there may be more interest in the surroundings and alertness, attention span, and communication skills may improve.[4] Many girls remain in this stage for most of their lives.[4]
Stage IV
Stage IV, or the late motor deterioration stage, can last for years or decades.[4] Prominent features include reduced mobility, curvature of the spine, and muscle weakness, rigidity, spasticity, and increased muscle tone with abnormal posturing of an arm, leg.[4] Girls who were previously able to walk may stop walking.[4] Cognition, communication, or hand skills generally do not decline in stage IV.[4] Repetitive hand movements may decrease and eye gaze usually improves.[4]
Variants
The signs of Rett syndrome typical form are perfectly identified (e.g. see above). In addition to the classical form of Rett syndrome, several atypical forms have been described over the years;[10] the main groups are:
- Congenital variant (Rolando variant): in this severe subtype of Rett syndrome, the development of the patients and their head circumference are abnormal from birth.[11] The typical gaze of Rett syndrome patients is usually absent;
- Zappella variant of Rett Syndrome or preserved speech variant: in this subtype of Rett syndrome the patients acquire some manual skills and language is partially recovered around the age of 5 years (that is after the regression phase). Height, weight and head circumference are often in the normal range, and a good gross motor function can be observed.[12][13][14][15][16][17] The Zappella variant is a milder form of Rett syndrome;
- Hanefeld variant or early epilepsy variant. In this form of Rett syndrome, the patients suffer from epilepsy before 5 months of age.[18]
The definition itself of the Rett syndrome has been refined over the years: as the atypical forms subsist near to the classical form (Hagberg & Gillberg, 1993), the "Rett Complex" terminology has been introduced.[19][20]
Cause
Genetically, Rett syndrome (RTT) is caused by mutations in the gene MECP2 located on the X chromosome (which is involved in transcriptional silencing and epigenetic regulation of methylated DNA), and can arise sporadically or from germline mutations. In less than 10% of RTT cases, mutations in the genes CDKL5 or FOXG1 have also been found to resemble it. Rett syndrome is initially diagnosed by clinical observation, but the diagnosis is definitive when there is a genetic defect in the MECP2 gene. In some very rare cases, no known mutated gene can be found; possibly due to changes in MECP2 that are not identified by presently used techniques or mutations in other genes that may result in clinical similarities.
It has been argued that Rett syndrome is in fact a neurodevelopmental condition as opposed to a neurodegenerative condition. One piece of evidence for this is that mice with induced Rett Syndrome show no neuronal death, and some studies have suggested that their phenotypes can be partially rescued by adding functional MECP2 gene back when they are adults. This information has also helped lead to further studies aiming to treat the disorder.[21]
Sporadic mutations
In at least 95% of Rett syndrome cases, the cause is a de novo mutation in the child. That is, it is not inherited from either parent. Parents are generally genotypically normal, without a MECP2 mutation.
In cases of the sporadic form of RTT, the mutated MECP2 is thought to derive almost exclusively from a de novo mutation on the male copy of the X chromosome.[22] It is not yet known what causes the sperm to mutate, and such mutations are rare.
Germline mutations
It can also be inherited from phenotypically normal mothers who have a germline mutation in the gene encoding methyl-CpG-binding protein-2, MeCP2.[23] In these cases, inheritance follows an X-linked dominant pattern and is seen almost exclusively in females, as most males die in utero or shortly after birth.[24] MECP2 is found near the end of the long arm of the X chromosome at Xq28. An atypical form of RTT, characterized by infantile spasms or early onset epilepsy, can also be caused by a mutation to the gene encoding cyclin-dependent kinase-like 5 (CDKL5). Rett syndrome affects one in every 12,500 female live births by age 12 years.
Mechanism
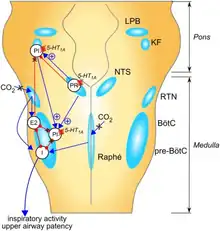
Pontine noradrenergic deficits
Brain levels of norepinephrine are lower in people with Rett syndrome[25] (reviewed in[26]). The genetic loss of MECP2 changes the properties of cells in the locus coeruleus, the exclusive source of noradrenergic innervation to the cerebral cortex and hippocampus.[27][28] These changes include hyperexcitability and decreased functioning of its noradrenergic innervation.[29] Moreover, a reduction of the tyrosine hydroxylase (Th) mRNA level, the rate-limiting enzyme in catecholamine synthesis, was detected in the whole pons of MECP2-null male as well as in adult heterozygous (MECP2+/-) female mice.[30] Using immunoquantitative techniques, a decrease of Th protein staining level, number of locus coeruleus TH-expressing neurons and density of dendritic arborization surrounding the structure was shown in symptomatic MeCP2-deficient mice.[30] However, locus coeruleus cells are not dying, but are more likely losing their fully mature phenotype, since no apoptotic neurons in the pons were detected.[30]
Researchers have concluded that "Because these neurons are a pivotal source of norepinephrine throughout the brainstem and forebrain and are involved in the regulation of diverse functions disrupted in Rett syndrome, such as respiration and cognition, we hypothesize that the locus coeruleus is a critical site at which loss of MECP2 results in CNS dysfunction." The restoration of normal locus coeruleus function may therefore be of potential therapeutic value in the treatment of Rett syndrome.[29][30]
Midbrain dopaminergic disturbances
The majority of dopamine in the mammalian brain is synthesized by nuclei located in the mesencephalon. The substantia nigra pars compacta (SNpc), the ventral tegmental area (VTA) and the retrorubral field (RRF) contains dopaminergic neurons expressing tyrosine hydroxylase (Th, i.e. the rate-limiting enzyme in catecholamine synthesis).[31][32][33]
The nigro-striatal pathway originates from SNpc and irradiate its principal rostral target, the Caudate-Putamen (CPu) through the median forebrain bundle (MFB). This connection is involved in the tight modulation of motor strategies computed by a cortico-basal ganglia- thalamo-cortical loop.[34]
Indeed, based on the canonical anatomofunctional model of basal ganglia, nigrostriatal dopamine is able to modulate the motor loop by acting on dopaminergic receptors located on striatal GABAergic medium spiny neurons.[35]
Dysregulation of the nigrostriatal pathway is causative from Parkinson disease (PD) in humans.[36] Toxic and/or genetic ablation of SNpc neurons produces experimental parkinsonism in mice and primates.[37] The common features of PD and PD animal models are motor impairments[38] (hypotonia, bradykinesia, hypokinesia).
RTT pathology, in some aspects, overlaps the motor phenotype observed in PD patients.[39][40][41] Several neuropathological studies on postmortem brain samples argued for an SNpc alteration evidenced by neuromelanin hypopigmentation, reduction in the structure area, and even controversial, signs of apoptosis. In parallel, an hypometabolism was underlined by a reduction of several catecholamines (dopamine, noradrenaline, adrenaline) and their principal metabolic by-products.[26] Mouse models of RTT are available and the most studied are constitutively deleted Mecp2 mice developed by Adrian Bird or Rudolf Jaenisch laboratories.[42][43][44][45]
In accordance with the motor spectrum of the RTT phenotype, Mecp2-null mice show motor abnormalities from postnatal day 30 that worsen until death. These models offer a crucial substrate to elucidate the molecular and neuroanatomical correlates of an MeCP2-deficiency.[46] Recently (2008), it was shown that the conditional deletion of Mecp2 in catecholaminergic neurons (by crossing of Th-Cre mice with loxP-flanked Mecp2 ones) recapitulates a motor symptomatology, it was further documented that brain levels of Th in mice lacking MeCP2 in catecholaminergic neurons only are reduced, participating to the motor phenotype.[47]
However, the most studied model for the evaluation of therapeutics is the Mecp2-null mouse (totally devoid of MeCP2). In this context, a reduction in the number and soma size of Th-expressing neurons is present from 5 weeks of age and is accompanied by a decrease of Th immunoreactivity in the caudate-putamen, the principal target of dopaminergic neurons arising from the SNpc.[48] Moreover, a neurochemical analysis of dopaminergic contents in microdissected midbrain and striatal areas revealed a reduction of dopamine at five and nine weeks of age. It is noteworthy that later on (at nine weeks), the morphological parameters remain altered but not worsen, whereas the phenotype progresses and behavioral deficits are more severe. The amount of fully activated Th (Serine40-phosphorylated isoform) in neurons that remain in the SNpc is mildly affected at 5 weeks but severely impaired by 9 weeks.[48] Finally, using a chronic and oral L-Dopa treatment on MeCP2-deficient mice authors reported an amelioration of some of the motor deficits previously identified.[48] Altogether, these results argue for an alteration of the nigrostriatal dopaminergic pathway in MeCP2-deficient animals as a contributor of the neuromotor deficits.
There is an association of the disease with brain-derived neurotrophic factor (BDNF).[49]
Interactive pathway map
An interactive pathway map of Rett syndrome has been published.[50]
Diagnosis

Prior to the discovery of a genetic cause, Rett syndrome had been designated as a pervasive developmental disorder by the Diagnostic and Statistical Manual of Mental Disorders (DSM), together with the autism spectrum disorders. Some argued against this conclusive assignment because RTT resembles non-autistic disorders such as fragile X syndrome, tuberous sclerosis, or Down syndrome that also exhibit autistic features.[51] After research proved the molecular mechanism, in 2013 the DSM-5 removed the syndrome altogether from classification as a mental disorder.[52]
Rett syndrome diagnosis involves close observation of the child's growth and development to observe any abnormalities in regards to developmental milestones.[53] A diagnosis is considered when decreased head growth is observed. Conditions with similar symptoms must first be ruled out.[53]
There is a certain criteria that must be met for the diagnosis. A blood test can rule in or rule out the presence of the MECP2 mutation, however, this mutation is present in other conditions as well.[54]
For a classic diagnosis, all four criteria for ruling in a diagnosis must be met, as well as the two criteria for ruling out a diagnosis. Supportive criteria may also be present, but are not required for diagnosis. For an atypical or variant diagnosis, at least two of the four criteria for ruling in the diagnosis must be met, as well as five of the eleven supportive criteria. A period of symptom regression followed by recovery or symptom stabilization must also occur.[54] Children are often misdiagnosed as having autism, cerebral palsy, or another form of developmental delay. A positive test for the MECP2 mutation is not enough to make a diagnosis.[54]
Ruling in[54]
- Decreased or loss of use of fine motor skills
- Decreased or loss of verbal speech
- Abnormalities during gait
- Repetitive hand movements such as wringing/squeezing or clapping/tapping
Ruling out[54]
- Traumatic brain injury, neurometabolic disease, or severe infection that may better explain symptoms
- Abnormal psychomotor development during the first six months of life
Supportive criteria[54]
- Breathing disturbances when awake
- Bruxism while awake
- Impaired sleep pattern
- Abnormal muscle tone
- Peripheral vasomotor disturbances
- Scoliosis/kyphosis
- Growth retardation
- Small cold hands and feet
- Inappropriate laughing/screaming spells
- Diminished response to pain
- Intense eye communication (eye pointing)
Differential diagnosis
Signs of Rett syndrome that are similar to autism:
- screaming fits
- inconsolable crying
- avoidance of eye contact
- lack of social/emotional reciprocity
- markedly impaired use of nonverbal behaviors to regulate social interaction
- loss of speech
- sensory problems
- sleep regression
Signs of Rett syndrome that are also present in cerebral palsy (regression of the type seen in Rett syndrome would be unusual in cerebral palsy; this confusion could rarely be made):
- possible short stature, sometimes with unusual body proportions because of difficulty walking or malnutrition caused by difficulty swallowing
- hypotonia
- delayed or absent ability to walk
- gait/movement difficulties
- ataxia
- microcephaly in some - abnormally small head, poor head growth
- gastrointestinal problems
- some forms of spasticity
- chorea - spasmodic movements of hand or facial muscles
- dystonia
- bruxism – grinding of teeth
Treatment
Currently there is no cure for Rett syndrome.[4] Treatment is directed towards improving function and addressing symptoms.[4] A multi-disciplinary team approach is typically used to treat the person throughout life. This team may include primary care physician, physical therapist, occupational therapist, speech-language pathologist, nutritionist, and support services in academic and occupational settings. Some children may require special equipment and aids such as braces to arrest scoliosis, splints to modify hand movements, and nutritional programs to help them maintain adequate weight.[4]
Treatment of Rett syndrome includes:
- management of gastrointestinal (reflux, constipation) and nutritional (poor weight gain) issues
- surveillance of scoliosis
- surveillance of long QT syndrome by annual EKG
- increasing the patient's communication skills, especially with augmentative communication strategies
- parental counseling
- modifying social medications
- sleep aids
- selective serotonin reuptake inhibitors (SSRIs)
- anti-psychotics (for self-harming behaviors)
- beta-blockers for long QT syndrome
- occupational therapy, speech therapy and physical therapy
Because of the increased risk of sudden cardiac death, when long QT syndrome is found on an annual screening EKG it is treated with an anti-arrhythmic such as a beta-blocker. There is some evidence that phenytoin may be more effective than a beta-blocker.[55]
Prognosis
Males with pathogenic MECP2 mutations usually die within the first 2 years from severe encephalopathy, unless they have one or more extra X chromosomes, or have somatic mosaicism.
Male fetuses with the disorder rarely survive to term. Because the disease-causing gene is located on the X chromosome, a female born with an MECP2 mutation on her X chromosome has another X chromosome with an ostensibly normal copy of the same gene, while a male with the mutation on his X chromosome has no other X chromosome, only a Y chromosome; thus, he has no normal gene. Without a normal gene to provide normal proteins in addition to the abnormal proteins caused by a MECP2 mutation, the XY karyotype male fetus is unable to slow the development of the disease, hence the failure of many male fetuses with a MECP2 mutation to survive to term.
Females with a MECP2 mutation, however, have a non-mutant chromosome that provides them enough normal protein to survive longer. Research shows that males with Rett syndrome may result from Klinefelter's syndrome, in which the male has an XXY karyotype.[56] Thus, a non-mutant MECP2 gene is necessary for a Rett's-affected embryo to survive in most cases, and the embryo, male or female, must have another X chromosome.
There have, however, been several cases of 46,XY karyotype males with a MECP2 mutation (associated with classical Rett syndrome in females) carried to term, who were affected by neonatal encephalopathy and died before 2 years of age.[57] The incidence of Rett syndrome in males is unknown, partly owing to the low survival of male fetuses with the Rett syndrome-associated MECP2 mutations, and partly to differences between signs caused by MECP2 mutations and those caused by Rett's.[57]
Females can live up to 40 years or more. Laboratory studies on Rett syndrome may show abnormalities such as:
- EEG abnormalities from 2 years of age
- atypical brain glycolipids
- elevated CSF levels of beta-endorphin and glutamate
- reduction of substance P
- decreased levels of CSF nerve growth factors
A high proportion of deaths are abrupt, but most have no identifiable cause; in some instances death is the result most likely of:
- spontaneous brainstem dysfunction
- cardiac arrest, likely due to long QT syndrome, ventricular tachycardia or other arrhythmias[58]
- seizures
- gastric perforation
History
Andreas Rett, a pediatrician in Vienna, first described the condition in 1966.[4][6] As his writings were in German, they did not become widely known in the English-speaking world.[7] Bengt Hagberg, a Swedish pediatrician, published an English article in 1983 and named the condition after Rett.[7] In 1999, Lebanese-American physician Huda Zoghbi discovered the mutation that causes the condition.[7][8]
Research
Gene therapy is under study in animal models to achieve regulated expression of a normal MECP2 gene.[4]
References
- ↑ Davis, Andrew S. (25 October 2010). Handbook of Pediatric Neuropsychology. Springer Publishing Company. ISBN 978-0826157362. Archived from the original on 5 November 2017.
Rett initially called this syndrome cerebroaatrophic hyperammonemia, but the elevated ammonia levels in the bloodstream were later found to be only rarely associated with this condition (can Acker, Loncola, & Can Acker, 2005).
- ↑ "MeSH Browser". meshb.nlm.nih.gov. Archived from the original on 4 December 2020. Retrieved 22 October 2019.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Rett syndrome". Genetics Home Reference. December 2013. Archived from the original on 14 October 2017. Retrieved 14 October 2017.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 "Rett Syndrome Fact Sheet". National Institute of Neurological Disorders and Stroke. Archived from the original on 14 October 2017. Retrieved 14 October 2017.
- ↑ "Rett Syndrome". NORD (National Organization for Rare Disorders). 2015. Archived from the original on 19 February 2017. Retrieved 14 October 2017.
- 1 2 Rett, A. (10 September 1966). "[On an unusual brain atrophy syndrome in hyperammonemia in childhood]". Wiener Medizinische Wochenschrift (in Deutsch). 116 (37): 723–726. ISSN 0043-5341. PMID 5300597.
- 1 2 3 4 5 6 Percy, Alan (January 2014). "The American History of Rett Syndrome". Pediatric Neurology. 50 (1): 1–3. doi:10.1016/j.pediatrneurol.2013.08.018. PMC 3874243. PMID 24200039.
- 1 2 Amir, Ruthie; Van den Veyver, Ignatia; Wan, Mimi; Tran, Charles; Francke, Uta; Zoghbi, Huda (1999). "Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2". Nature Genetics. 23 (2): 185–8. doi:10.1038/13810. PMID 10508514. S2CID 3350350.
- ↑ Fuertes-González, MC; Silvestre, FJ (1 November 2014). "Oral health in a group of patients with Rett syndrome in the regions of Valencia and Murcia (Spain): a case-control study". Medicina Oral, Patologia Oral y Cirugia Bucal. 19 (6): e598-604. doi:10.4317/medoral.19743. PMC 4259377. PMID 25350594.
- ↑ Neul, Jeffrey l.; Kaufmann, Walter E.; Glaze, Daniel G.; Christodoulou, John; Clarke, Angus J.; Bahi-Buisson, Nadia; Leonard, Helen; Bailey, Mark E. S.; Schanen, N. Carolyn; Zappella, Michele; Renieri, Alessandra; Huppke, Peter; Percy, Alan K.; et al. (Rettsearch Consortium) (2010). "Rett syndrome: Revised diagnostic criteria and nomenclature". Annals of Neurology. 68 (6): 944–50. doi:10.1002/ana.22124. PMC 3058521. PMID 21154482.
- ↑ Ariani, Francesca; Hayek, Giuseppe; Rondinella, Dalila; Artuso, Rosangela; Mencarelli, Maria Antonietta; Spanhol-Rosseto, Ariele; Pollazzon, Marzia; Buoni, Sabrina; Spiga, Ottavia; Ricciardi, Sara; Meloni, Ilaria; Longo, Ilaria; Mari, Francesca; Broccoli, Vania; Zappella, Michele; Renieri, Alessandra (11 July 2008). "FOXG1 is Responsible for the Congenital Variant of Rett Syndrome". The American Journal of Human Genetics. 83 (1): 89–93. doi:10.1016/j.ajhg.2008.05.015. PMC 2443837. PMID 18571142.
- ↑ Zappella, Michele (1992). "The rett girls with preserved speech". Brain and Development. 14 (2): 98–101. doi:10.1016/S0387-7604(12)80094-5. PMID 1621933. S2CID 4782923.
- ↑ Skjeldal, O. H.; Von Tetzchner, S.; Jacobsen, K.; Smith, L.; Heiberg, A. (2007). "Rett Syndrome - Distribution of Phenotypes with Special Attention to the Preserved Speech Variant". Neuropediatrics. 26 (2): 87. doi:10.1055/s-2007-979732. PMID 7566462.
- ↑ Sørensen, E.; Viken, B. (1995-02-20). "[Rett syndrome a developmental disorder. Presentation of a variant with preserved speech]". Tidsskrift for den Norske Laegeforening: Tidsskrift for Praktisk Medicin, NY Raekke (in norsk). 115 (5): 588–590. ISSN 0029-2001. PMID 7900110.
- ↑ Zappella, M (1997). "The preserved speech variant of the Rett complex: A report of 8 cases". European Child & Adolescent Psychiatry. 6 Suppl 1: 23–5. PMID 9452915.
- ↑ Renieri, A.; Mari, F.; Mencarelli, M.A.; Scala, E.; Ariani, F.; Longo, I.; Meloni, I.; Cevenini, G.; Pini, G.; Hayek, G.; Zappella, M. (March 2009). "Diagnostic criteria for the Zappella variant of Rett syndrome (the preserved speech variant)". Brain and Development. 31 (3): 208–16. doi:10.1016/j.braindev.2008.04.007. PMID 18562141. S2CID 6223422.
- ↑ Buoni, Sabrina; Zannolli, Raffaella; De Felice, Claudio; De Nicola, Anna; Guerri, Vanessa; Guerra, Beatrice; Casali, Stefania; Pucci, Barbara; Corbini, Letizia; Mari, Francesca; Renieri, Alessandra; Zappella, Michele; Hayek, Joseph (May 2010). "EEG features and epilepsy in MECP2-mutated patients with the Zappella variant of Rett syndrome". Clinical Neurophysiology. 121 (5): 652–7. doi:10.1016/j.clinph.2010.01.003. PMID 20153689. S2CID 12976926.
- ↑ Huppke, Peter; Held, Melanie; Laccone, Franco; Hanefeld, Folker (2003). "The spectrum of phenotypes in females with Rett Syndrome". Brain and Development. 25 (5): 346–51. doi:10.1016/S0387-7604(03)00018-4. PMID 12850514. S2CID 9566219.
- ↑ Gillberg, C. (1997). "Communication in Rett syndrome complex". European Child & Adolescent Psychiatry. 6 Suppl 1: 21–2. PMID 9452914.
- ↑ Zappella, Michele; Gillberg, Christopher; Ehlers, Stephan (1998). "The preserved speech variant: A subgroup of the Rett complex: A clinical report of 30 cases". Journal of Autism and Developmental Disorders. 28 (6): 519–26. doi:10.1023/A:1026052128305. PMID 9932238. S2CID 22152062.
- ↑ Guy, J.; Gan, J.; Selfridge, J.; Cobb, S.; Bird, A. (2007). "Reversal of Neurological Defects in a Mouse Model of Rett Syndrome". Science. 315 (5815): 1143–7. Bibcode:2007Sci...315.1143G. doi:10.1126/science.1138389. PMID 17289941.
- ↑ Trappe, R.; Laccone, F.; Cobilanschi, J.; Meins, M.; Huppke, P.; Hanefeld, F.; Engel, W. (2001). "MECP2 Mutations in Sporadic Cases of Rett Syndrome Are Almost Exclusively of Paternal Origin". The American Journal of Human Genetics. 68 (5): 1093–101. doi:10.1086/320109. PMC 1226090. PMID 11309679.
- ↑ Zoghbi, Huda Y.; Van Den Veyver, Ruthie E.; Wan, Ignatia B.; Tran, Mimi; Francke, Charles Q.; Zoghbi, Uta (1999). "Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2". Nature Genetics. 23 (2): 185–8. doi:10.1038/13810. PMID 10508514. S2CID 3350350.
- ↑ "Rett syndrome". Genetics Home Reference. Archived from the original on 2016-07-27. Retrieved 2016-05-29.
- ↑ Zoghbi, Huda Y.; Milstien, Sheldon; Butler, Ian J.; Smith, E. O'Brian; Kaufman, Seymour; Glaze, Daniel G.; Percy, Alan K. (1989). "Cerebrospinal fluid biogenic amines and biopterin in Rett syndrome". Annals of Neurology. 25 (1): 56–60. doi:10.1002/ana.410250109. PMID 2913929.
- 1 2 Roux, Jean-Christophe; Villard, Laurent (2009). "Biogenic Amines in Rett Syndrome: The Usual Suspects". Behavior Genetics. 40 (1): 59–75. doi:10.1007/s10519-009-9303-y. PMID 19851857. S2CID 20352177.
- ↑ Hokfelt, T.; Martensson, R.; Bjorklund, A.; Kleinau, S.; Goldstein, M (1984). "Distribution maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain". In Bjorklund, A.; Hokfelt, T. (eds.). Handbook of Chemical Neuroanatomy. Classical Transmitters in the CNS, Part I. Vol. 2. New York: Elsevier. pp. 277–379.
- ↑ Berridge, Craig W; Waterhouse, Barry D (2003). "The locus coeruleus–noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes". Brain Research Reviews. 42 (1): 33–84. doi:10.1016/S0165-0173(03)00143-7. PMID 12668290. S2CID 477754.
- 1 2 Taneja, P.; Ogier, M.; Brooks-Harris, G.; Schmid, D. A.; Katz, D. M.; Nelson, S. B. (2009). "Pathophysiology of Locus Ceruleus Neurons in a Mouse Model of Rett Syndrome". Journal of Neuroscience. 29 (39): 12187–95. doi:10.1523/JNEUROSCI.3156-09.2009. PMC 2846656. PMID 19793977.
- 1 2 3 4 Roux, Jean-Christophe; Panayotis, Nicolas; Dura, Emmanuelle; Villard, Laurent (2009). "Progressive noradrenergic deficits in the locus coeruleus of Mecp2 deficient mice". Journal of Neuroscience Research. 88 (7): 1500–9. doi:10.1002/jnr.22312. PMID 19998492.
- ↑ Björklund, A.; Lindvall, O (1984). "Dopamine-containing systems in the CNS". In Björklund, A.; Hökfelt, T. (eds.). Handbook of Chemical Neuroanatomy. Classical Transmitters in the CNS, Part l. Vol. 2. New York: Elsevier. pp. 55–122.
- ↑ Hokfelt, T.; Martensson, R.; Björklund, A.; Kleinau, S.; Goldstein, M. (1984). "Distribution maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain". In Björklund, A.; Hökfelt, T. (eds.). Handbook of Chemical Neuroanatomy. Classical Transmitters in the CNS, Part I. Vol. 2. New York: Elsevier. pp. 277–379.
- ↑ Björklund, Anders; Dunnett, Stephen B. (2007). "Dopamine neuron systems in the brain: An update". Trends in Neurosciences. 30 (5): 194–202. doi:10.1016/j.tins.2007.03.006. PMID 17408759. S2CID 14239716.
- ↑ Parent, André; Hazrati, Lili-Naz (1995). "Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop". Brain Research Reviews. 20 (1): 91–127. doi:10.1016/0165-0173(94)00007-C. PMID 7711769. S2CID 28252990.
- ↑ Gerfen, Charles R. (2000). "Molecular effects of dopamine on striatal-projection pathways". Trends in Neurosciences. 23 (10 Suppl): S64–70. doi:10.1016/S1471-1931(00)00019-7. PMID 11052222. S2CID 3965480.
- ↑ Lees, Andrew J; Hardy, John; Revesz, Tamas (2009). "Parkinson's disease". The Lancet. 373 (9680): 2055–66. doi:10.1016/S0140-6736(09)60492-X. PMID 19524782. S2CID 42608600.
- ↑ Dauer, William; Przedborski, Serge (2003). "Parkinson's Disease". Neuron. 39 (6): 889–909. doi:10.1016/S0896-6273(03)00568-3. PMID 12971891. S2CID 10400095.
- ↑ Jenner, Peter (2009). "Functional models of Parkinson's disease: A valuable tool in the development of novel therapies". Annals of Neurology. 64: S16–29. doi:10.1002/ana.21489. PMID 19127585.
- ↑ Fitzgerald, Patricia M.; Jankovic, Joseph; Percy, Alan K. (1990). "Rett syndrome and associated movement disorders". Movement Disorders. 5 (3): 195–202. doi:10.1002/mds.870050303. PMID 2388636.
- ↑ Neul, Jeffrey L.; Zoghbi, Huda Y. (2004). "Rett Syndrome: A Prototypical Neurodevelopmental Disorder". The Neuroscientist. 10 (2): 118–28. doi:10.1177/1073858403260995. PMID 15070486. S2CID 9617631.
- ↑ Segawa, Masaya (2005). "Early motor disturbances in Rett syndrome and its pathophysiological importance". Brain and Development. 27: S54–S58. doi:10.1016/j.braindev.2004.11.010. PMID 16182486. S2CID 30218744.
- ↑ Guy, Jacky; Hendrich, Brian; Holmes, Megan; Martin, Joanne E.; Bird, Adrian (2001). "A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome". Nature Genetics. 27 (3): 322–6. doi:10.1038/85899. hdl:1842/727. PMID 11242117. S2CID 8698208.
- ↑ Chen, Richard Z.; Akbarian, Schahram; Tudor, Matthew; Jaenisch, Rudolf (2001). "Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice". Nature Genetics. 27 (3): 327–31. doi:10.1038/85906. PMID 11242118. S2CID 24979562.
- ↑ Nan, X; Ng, H. H.; Johnson, C. A.; Laherty, C. D.; Turner, B. M.; Eisenman, R. N.; Bird, A (1998). "Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex". Nature. 393 (6683): 386–9. Bibcode:1998Natur.393..386N. doi:10.1038/30764. PMID 9620804. S2CID 4427745.
- ↑ Cheval, H; Guy, J; Merusi, C; De Sousa, D; Selfridge, J; Bird, A (2012). "Postnatal inactivation reveals enhanced requirement for MeCP2 at distinct age windows". Human Molecular Genetics. 21 (17): 3806–14. doi:10.1093/hmg/dds208. PMC 3412380. PMID 22653753.

- ↑ Ricceri, Laura; De Filippis, Bianca; Laviola, Giovanni (2008). "Mouse models of Rett syndrome: From behavioural phenotyping to preclinical evaluation of new therapeutic approaches". Behavioural Pharmacology. 19 (5–6): 501–17. doi:10.1097/FBP.0b013e32830c3645. PMID 18690105. S2CID 33364486.
- ↑ Samaco, R. C.; Mandel-Brehm, C.; Chao, H.-T.; Ward, C. S.; Fyffe-Maricich, S. L.; Ren, J.; Hyland, K.; Thaller, C.; Maricich, S. M.; Humphreys, P.; Greer, J. J.; Percy, A.; Glaze, D. G.; Zoghbi, H. Y.; Neul, J. L. (2009). "Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities". Proceedings of the National Academy of Sciences. 106 (51): 21966–71. Bibcode:2009PNAS..10621966S. doi:10.1073/pnas.0912257106. JSTOR 40536204. PMC 2799790. PMID 20007372.
- 1 2 3 Panayotis, Nicolas; Pratte, Michel; Borges-Correia, Ana; Ghata, Adeline; Villard, Laurent; Roux, Jean-Christophe (2011). "Morphological and functional alterations in the substantia nigra pars compacta of the Mecp2-null mouse". Neurobiology of Disease. 41 (2): 385–97. doi:10.1016/j.nbd.2010.10.006. PMID 20951208. S2CID 25414717.
- ↑ Sun, Yi E.; Wu, Hao (2006). "The Ups and Downs of BDNF in Rett Syndrome". Neuron. 49 (3): 321–3. doi:10.1016/j.neuron.2006.01.014. PMID 16446133.
- ↑ Ehrhart, Friederike; Coort, Susan L. M.; Cirillo, Elisa; Smeets, Eric; Evelo, Chris T.; Curfs, Leopold M. G. (25 November 2016). "Rett syndrome – biological pathways leading from MECP2 to disorder phenotypes". Orphanet Journal of Rare Diseases. 11 (1): 158. doi:10.1186/s13023-016-0545-5. PMC 5123333. PMID 27884167.
- ↑ Tsai, Luke Y. (1992). "Is Rett syndrome a subtype of pervasive developmental disorders?" (PDF). Journal of Autism and Developmental Disorders. 22 (4): 551–61. doi:10.1007/BF01046327. hdl:2027.42/44607. PMID 1483976. S2CID 17817425. Archived from the original on 29 August 2021. Retrieved 20 April 2018.
- ↑ Abbeduto, Leonard; Ozonoff, Susan; Thurman, Angela John; McDuffie, Angela; Schweitzer, Julie (18 March 2014). Hales, Robert; Yudofsky, Stuart; Robert, Laura Weiss (eds.). Chapter 8. Neurodevelopmental Disorders, The American Psychiatric Publishing Textbook of Psychiatry (6 ed.). Arlington, VA: American Psychiatric Publishing. doi:10.1176/appi.books.9781585625031.rh08. ISBN 978-1-58562-444-7.
- 1 2 "Rett syndrome Tests and diagnosis". Mayo Clinic. Archived from the original on 2017-10-30.
- 1 2 3 4 5 6 "About Rett syndrome - Rett Syndrome Diagnosis". www.rettsyndrome.org. International Rett Syndrome Foundation. Archived from the original on 2017-10-29. Retrieved 10 May 2020.
- ↑ McCauley, Mark D.; Wang, Tiannan; Mike, Elise; Herrera, Jose; Beavers, David L.; Huang, Teng-Wei; Ward, Christopher S.; Skinner, Steven; Percy, Alan K. (2011-12-14). "Pathogenesis of Lethal Cardiac Arrhythmias in Mecp2 Mutant Mice: Implication for Therapy in Rett Syndrome". Science Translational Medicine. 3 (113): 113ra125. doi:10.1126/scitranslmed.3002982. ISSN 1946-6234. PMC 3633081. PMID 22174313.
- ↑ Schwartzman, J. S.; Bernardino, Andrea; Nishimura, Agnes; Gomes, Raquel R.; Zatz, Mayana (2001). "Rett Syndrome in a Boy with a 47,XXY Karyotype Confirmed by a Rare Mutation in the MECP2 Gene". Neuropediatrics. 32 (3): 162–4. doi:10.1055/s-2001-16620. PMID 11521215.
- 1 2 Hardwick, Simon A; Reuter, Kirsten; Williamson, Sarah L; Vasudevan, Vidya; Donald, Jennifer; Slater, Katrina; Bennetts, Bruce; Bebbington, Ami; Leonard, Helen; Williams, Simon R; Smith, Robert L; Cloosterman, Desiree; Christodoulou, John (2007). "Delineation of large deletions of the MECP2 gene in Rett syndrome patients, including a familial case with a male proband". European Journal of Human Genetics. 15 (12): 1218–29. doi:10.1038/sj.ejhg.5201911. PMID 17712354.
- Lay summary in: "New Study Reveals Rett Syndrome Can Strike Males". ScienceDaily. August 12, 2006.
- ↑ Acampa, M.; Guideri, F. (May 2006). "Cardiac disease and Rett syndrome". Archives of Disease in Childhood. 91 (5): 440–443. doi:10.1136/adc.2005.090290. ISSN 1468-2044. PMC 2082747. PMID 16632674.
External links
| Classification | |
|---|---|
| External resources |
|