Odevixibat
 | |
| Names | |
|---|---|
| Trade names | Bylvay |
| Other names | A4250 |
IUPAC name
| |
| Clinical data | |
| Side effects | Diarrhea, abdominal pain, liver enlargement, fat-soluble vitamin deficiency, liver inflammation[1][2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
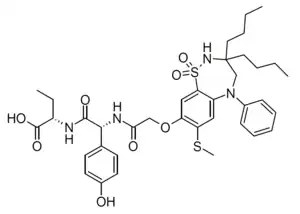
| Formula | C37H48N4O8S2 |
| Molar mass | 740.93 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Odevixibat, sold under the trade name Bylvay, is a medication used to treat itchiness in progressive familial intrahepatic cholestasis (PFIC).[2] It is used in those who are at least 3 months old.[2] It is taken by mouth.[2]
Common side effects include diarrhea, abdominal pain, liver enlargement, fat-soluble vitamin deficiency, and liver inflammation.[1][2] Safety in pregnancy is unclear, with some evidence of harm.[2] It is a inhibitor of the ileal bile acid transporter (IBAT).[2]
Odevixibat was approved for medical use in the United States and Europe in 2021.[2][1] In the United Kingdom a month of medication at a dose of 400 mcg is about £6,200 as of 2022.[4] This amount in the United States is about 13,000 USD.[5]
Medical uses
In the United States, it is used for itchiness in people three months of age and older with progressive familial intrahepatic cholestasis (PFIC).[2] In the European Union it is indicated in people six months of age and older.[1][3][6] It might not work in PFIC type 2 with ABCB11 variants resulting in non-functional or complete absence of bile salt export pump protein (BSEP-3).[2]
Dosage
It is generally taken at a dose of 40 mcg/kg per day which may be increased up to 120 mcg/kg per day with a maximum dose of 6 mg.[2]
Side effects
Common side effects include diarrhea, stomach pain, vomiting, abnormal liquid function tests, and a deficiency in vitamins A,D, E and K. [7]
Odevixibat cannot be given to a child on a liquid diet.[8]
Mechanism of action
Odevixibat is a reversible inhibitor of the ileal sodium/bile acid transporter which is the transporter responsible for reabsorption of the majority of bile acids in the distal ileum. [9] The reduced absorption of the bile acids in the distal ileum compounds and leads to a decrease in stimulation of FXR, decreasing the inhibition of bile acid synthesis.[7]
Pharmacokinetics
Odevixibat is majorly protein-bound in-vitro.[7] A dose of 7.2 mg reaches a Cmax concentration of 0.47 ng/mL with an AUC (0-24h) of 2.19 h*ng/mL. [8] Adult and childreb given the therapeautic dose did not display plasma concentrations of the drug.[10] Odevixibat is eliminated majorly unchanged.[8] Odevixibat has an average half-life of 2.36 hours.[7]
Society and culture
Legal status
In May 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended granting a marketing authorization in the European Union for odevixibat for the treatment of PFIC in people aged six months or older.[11][12] It was approved for medical use in the European Union in July 2021.[1][3]
References
- 1 2 3 4 5 6 "Bylvay EPAR". European Medicines Agency (EMA). 20 April 2021. Archived from the original on 29 July 2021. Retrieved 28 July 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 "Bylvay- odevixibat capsule, coated pellets". DailyMed. Archived from the original on 29 July 2021. Retrieved 28 July 2021.
- 1 2 3 "Bylvay". Union Register of medicinal products. Archived from the original on 24 July 2021. Retrieved 23 July 2021.
- ↑ "Odevixibat". SPS - Specialist Pharmacy Service. 17 December 2018. Archived from the original on 3 March 2022. Retrieved 29 October 2022.
- ↑ "Bylvay". Retrieved 29 October 2022.
- ↑ "Odevixibat: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 27 September 2021. Retrieved 23 July 2021.
- 1 2 3 4 "Odevixibat Uses, Side Effects & Warnings". Drugs.com. Archived from the original on 2022-06-11. Retrieved 2022-06-11.
- 1 2 3 "Odevixibat Uses, Side Effects & Warnings". Drugs.com. Archived from the original on 2022-06-11. Retrieved 2022-06-11.
- ↑ "Odevixibat". go.drugbank.com. Archived from the original on 2021-09-19. Retrieved 2022-06-13.
- ↑ "Albireo Announces FDA Approval of Bylvay (odevixibat), the First Drug Treatment for Patients With Progressive Familial Intrahepatic Cholestasis (PFIC)". Albireo Pharma (Press release). 20 July 2021. Archived from the original on 24 July 2021. Retrieved 23 July 2021 – via GlobeNewswire.
- ↑ "First treatment for rare liver disease". European Medicines Agency (EMA) (Press release). 21 May 2021. Archived from the original on 21 May 2021. Retrieved 21 May 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Bylvay: Pending EC decision". European Medicines Agency (EMA). 19 May 2021. Archived from the original on 21 May 2021. Retrieved 21 May 2021.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- Clinical trial number NCT03566238 for "This Study Will Investigate the Efficacy and Safety of A4250 in Children With PFIC 1 or 2 (PEDFIC 1)" at ClinicalTrials.gov