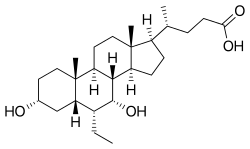
Obeticholic acid
 | |
| Names | |
|---|---|
| Trade names | Ocaliva |
| Other names | 6α-ethyl-chenodeoxycholic acid; INT-747 |
IUPAC name
| |
| Clinical data | |
| Drug class | Modified bile acid[1] |
| Main uses | Primary biliary cholangitis[2] |
| Side effects | Itching, tiredness, liver problems[2][1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Typical dose | 5 mg/wk to 10 mg/day[2][1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a616033 |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C26H44O4 |
| Molar mass | 420.634 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 108–110 °C (226–230 °F) [3] |
SMILES
| |
InChI
| |
Obeticholic acid (OCA), sold under the brand name Ocaliva, is a medication used to treat primary biliary cholangitis.[2] It is generally used in addition to ursodeoxycholic acid when this is insufficiently effective.[2] It is taken by mouth.[2]
Common side effects include itching and tiredness.[1] Other side effects may include liver problems.[2] It is a modified form of bile acid and works by attaching to farnesoid X receptors and decreasing bile production by the liver.[1]
Obeticholic acid was approved for medical use in the United States and Europe in 2016.[4][1] In the United Kingdom it costs the NHS about £2,400 per month as of 2021.[2] In the United States this amount costs about 8,000 USD.[5]
Medical uses
Primary biliary cholangitis
Primary biliary cholangitis (PBC), also known as primary biliary cirrhosis, is an auto-immune, inflammatory liver disease which produces bile duct injury, fibrosis, cholestasis and eventual cirrhosis.[6] It is much more common in women than men and can cause jaundice, itching (pruritus) and fatigue. Ursodeoxycholic acid therapy is beneficial, but the disease often progresses and may require liver transplantation.[7] Animal studies suggested that treatment with FXR agonists should be beneficial in cholestatic diseases such as PBC.[8] OCA at doses between 10 mg and 50 mg was shown to provide significant biochemical benefit, but pruritus was more frequent with higher doses.[9][10] The results of a randomized, double-blind phase III study of OCA, 5 mg or 10 mg, compared to placebo (POISE) were presented in April 2014, and showed that the drug met the trial's primary endpoint of a significant reduction in serum alkaline phosphatase, a biomarker predictive of disease progression, liver transplantation or death.[11]
Dosage
It is taken at a dose of 5 mg per day, which may be increased to 10 mg per day.[2] A dose of 5 mg once per week may be used in those with certain degrees of liver problems.[1]
History
The natural bile acid chenodeoxycholic acid was identified in 1999 as the most active physiological ligand for the farnesoid X receptor (FXR), which is involved in many physiological and pathological processes. A series of alkylated bile acid analogues were designed, studied and patented by Roberto Pellicciari and colleagues at the University of Perugia, with 6α-ethyl-chenodeoxycholic acid emerging as the most highly potent FXR agonist.[12] FXR-dependent processes in liver and intestine were proposed as therapeutic targets in human diseases.[13] Obeticholic acid is the first FXR agonist to be used in human drug studies.
The U.S. Food and Drug Administration (FDA) approved obeticholic acid on May 27, 2016, for the treatment of primary biliary cholangitis. It was approved as an orphan drug based on its reduction in the level of the biomarker alkaline phosphatase as a surrogate endpoint for clinical benefit.[14] It is indicated for the treatment of primary biliary cholangitis in combination with ursodeoxycholic acid in adults with an inadequate response to UDCA, or as monotherapy in adults unable to tolerate UDCA.[15] Additional studies are being required to prove its clinical benefit.[16]
Society and culture
Intercept Pharmaceuticals Inc. hold the worldwide rights to develop OCA outside Japan and China, where it is licensed to Dainippon Sumitomo Pharma.[17]
Research
Obeticholic acid is being studied for a number of liver and gastrointestinal conditions.[18]
Nonalcoholic steatohepatitis
Non-alcoholic steatohepatitis (NASH) OCA is proposed treatment.[19] A trial was halted early in January 2014, after about half of the 283 subjects had completed the study, when a planned interim analysis showed that a) the primary endpoint had been met and b) lipid abnormalities were detected and arose safety concerns. Treatment with OCA (25 mg/day for 72 weeks) resulted in a highly statistically significant improvement in the primary histological endpoint, defined as a decrease in the NAFLD Activity Score of at least two points, with no worsening of fibrosis. 45% (50 of 110) of the treated group had this improvement compared with 21% (23 of 109) of the placebo-treated controls.[20] However concerns about longterm safety issues such as increased cholesterol and adverse cardiovascular events may warrant the concomitant use of statins in OCA-treated patients.[21]
Portal hypertension
Animal studies suggest that OCA improves intrahepatic vascular resistance and so may be of therapeutic benefit in portal hypertension.[22] An open label phase IIa clinical study is under way.
Bile acid diarrhea
Bile acid diarrhea (also called bile acid malabsorption) can be secondary to Crohn's disease or be a primary condition. Reduced median levels of FGF19, an ileal hormone that regulates increased hepatic bile acid synthesis, have been found in this condition.[23] FGF19 is potently stimulated by bile acids and especially by OCA.[24] A proof of concept study of OCA (25 mg/d) has shown clinical and biochemical benefit.[25]
References
- 1 2 3 4 5 6 7 "Ocaliva". Archived from the original on 8 November 2021. Retrieved 6 November 2021.
- 1 2 3 4 5 6 7 8 9 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 96. ISBN 978-0857114105.
- ↑ Gioiello, Antimo; Macchiarulo, Antonio; Carotti, Andrea; Filipponi, Paolo; Costantino, Gabriele; Rizzo, Giovanni; Adorini, Luciano; Pellicciari, Roberto (April 2011). "Extending SAR of bile acids as FXR ligands: Discovery of 23-N-(carbocinnamyloxy)-3α,7α-dihydroxy-6α-ethyl-24-nor-5β-cholan-23-amine". Bioorganic & Medicinal Chemistry. 19 (8): 2650–2658. doi:10.1016/j.bmc.2011.03.004. PMID 21459580.
- ↑ "Obeticholic Acid Monograph for Professionals". Drugs.com. Archived from the original on 16 July 2017. Retrieved 6 November 2021.
- ↑ "Ocaliva Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 23 April 2021. Retrieved 6 November 2021.
- ↑ "Cirrhosis". The Lecturio Medical Concept Library. Archived from the original on 9 July 2021. Retrieved 9 July 2021.
- ↑ Hirschfield GM, Gershwin ME (January 2013). "The immunobiology and pathophysiology of primary biliary cirrhosis". Annu Rev Pathol. 8: 303–30. doi:10.1146/annurev-pathol-020712-164014. PMID 23347352.
- ↑ Lindor, KD (May 2011). "Farnesoid X receptor agonists for primary biliary cirrhosis". Current Opinion in Gastroenterology. 27 (3): 285–8. doi:10.1097/MOG.0b013e32834452c8. PMID 21297469. S2CID 43806943.
- ↑ Fiorucci S, Cipriani S, Mencarelli A, Baldelli F, Bifulco G, Zampella A (August 2011). "Farnesoid X receptor agonist for the treatment of liver and metabolic disorders: focus on 6-ethyl-CDCA". Mini Rev Med Chem. 11 (9): 753–62. doi:10.2174/138955711796355258. PMID 21707532.
- ↑ Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC, Parés A, Trauner M, Marschall HU, Adorini L, Sciacca C, Beecher-Jones T, Castelloe E, Böhm O, Shapiro D (2015). "Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid". Gastroenterology. 148 (4): 751–61.e8. doi:10.1053/j.gastro.2014.12.005. PMID 25500425.
- ↑ Intercept Pharma. "Press release: Intercept Announces Positive Pivotal Phase 3 POISE Trial Results". Archived from the original on March 26, 2014. Retrieved March 27, 2014.
- ↑ Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM (August 2002). "6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity". J. Med. Chem. 45 (17): 3569–72. doi:10.1021/jm025529g. PMID 12166927.
- ↑ Rizzo G, Renga B, Mencarelli A, Pellicciari R, Fiorucci S (September 2005). "Role of FXR in regulating bile acid homeostasis and relevance for human diseases". Curr. Drug Targets Immune Endocr. Metabol. Disord. 5 (3): 289–303. doi:10.2174/1568008054863781. PMID 16178789.
- ↑ "FDA Approves Ocaliva for Rare, Chronic Liver Disease" (Press release). U.S. Food and Drug Administration (FDA). May 31, 2016. Archived from the original on 10 November 2016. Retrieved 15 November 2016.
- ↑ "Ocaliva- obeticholic acid tablet, film coated". DailyMed. Archived from the original on 28 November 2020. Retrieved 17 December 2020.
- ↑ Egan, Amy G.(Deputy Director, Center for Drug Evaluation and Research). "Letter from Food and Drug Administration to Intercept Pharmaceuticals" (PDF). U.S. Food and Drug Administration (FDA). Archived (PDF) from the original on 15 November 2016. Retrieved 15 November 2016.
- ↑ The Wall Street Journal. "A $4 Billion Surprise for 45-Person Biotech". Archived from the original on 28 March 2014. Retrieved 10 January 2014.
- ↑ "ClinicalTrials.gov". Archived from the original on 2016-03-06. Retrieved 2021-10-24.
- ↑ Adorini L, Pruzanski M, Shapiro D (September 2012). "Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis". Drug Discov. Today. 17 (17–18): 988–97. doi:10.1016/j.drudis.2012.05.012. PMID 22652341.
- ↑ Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E (2015). "Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial". Lancet. 385 (9972): 956–65. doi:10.1016/S0140-6736(14)61933-4. PMC 4447192. PMID 25468160.
- ↑ "Intercept Pharma, Government Scientists Spar over Negative Safety of Liver Drug, Emails Show". 2014-05-20. Archived from the original on 2015-04-02. Retrieved 2021-10-24.
- ↑ Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, Vander Elst I, Windmolders P, Vanuytsel T, Nevens F, Laleman W (November 2013). "Obeticholic acid, a farnesoid-X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats". Hepatology. 59 (6): 2286–98. doi:10.1002/hep.26939. PMID 24259407. S2CID 205891475.
- ↑ Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW (November 2009). "A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis". Clin. Gastroenterol. Hepatol. 7 (11): 1189–94. doi:10.1016/j.cgh.2009.04.024. PMID 19426836. Archived from the original on 2020-04-20. Retrieved 2021-10-24.
- ↑ Zhang JH, Nolan JD, Kennie SL, Johnston IM, Dew T, Dixon PH, Williamson C, Walters JR (May 2013). "Potent stimulation of fibroblast growth factor 19 expression in the human ileum by bile acids". Am. J. Physiol. Gastrointest. Liver Physiol. 304 (10): G940–8. doi:10.1152/ajpgi.00398.2012. PMC 3652069. PMID 23518683.
- ↑ Walters JR, Johnston IM, Nolan JD, Vassie C, Pruzanski ME, Shapiro DA (January 2015). "The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid". Aliment. Pharmacol. Ther. 41 (1): 54–64. doi:10.1111/apt.12999. hdl:10044/1/21617. PMID 25329562. S2CID 44661338.
External links
| Identifiers: |
|---|
- "Obeticholic acid". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-10-25. Retrieved 2021-10-24.