Ritlecitinib
 | |
| Clinical data | |
|---|---|
| Trade names | Litfulo |
| Other names | PF-06651600 |
| License data |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
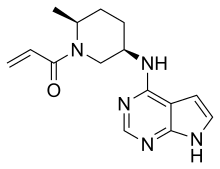
| Formula | C15H19N5O |
| Molar mass | 285.351 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ritlecitinib, sold under the brand name Litfulo, is a medication used for the treatment of severe alopecia areata (hair loss).[1] Ritlecitinib is a kinase inhibitor which inhibits Janus kinase 3 and tyrosine kinase.[1][4][5]
Ritlecitinib was approved for medical use in the United States in June 2023,[1][6] and the European Union in September 2023.[2]
Medical uses
Ritlecitinib is indicated for the treatment of severe alopecia areata for individuals aged 12 and over.[1][2]
Society and culture
Economics
The annual list price of a prescription is $49,000. [7]
References
- 1 2 3 4 5 "Litfulo- ritlecitinib capsule". DailyMed. U.S. National Library of Medicine. 23 June 2023. Archived from the original on 29 August 2023. Retrieved 28 August 2023.
- 1 2 3 "Litfulo EPAR". European Medicines Agency. 18 September 2023. Archived from the original on 19 September 2023. Retrieved 20 September 2023.
- ↑ "Litfulo Product information". Union Register of medicinal products. 18 September 2023. Archived from the original on 1 October 2023. Retrieved 1 October 2023.
- ↑ "Ritlecitinib". Inxight Drugs. Archived from the original on 25 June 2023. Retrieved 24 June 2023.
- ↑ Ramírez-Marín HA, Tosti A (February 2022). "Evaluating the Therapeutic Potential of Ritlecitinib for the Treatment of Alopecia Areata". Drug Design, Development and Therapy. 16: 363–374. doi:10.2147/DDDT.S334727. PMC 8860347. PMID 35210753.
- ↑ "FDA Approves Pfizer's Litfulo (Ritlecitinib) for Adults and Adolescents With Severe Alopecia Areata" (Press release). Pfizer. 23 June 2023. Archived from the original on 25 June 2023. Retrieved 24 June 2023 – via Business Wire.
- ↑ Kansteiner F (26 June 2023). "Pfizer's Litfulo enters the scene in alopecia with adolescent nod to rival Lilly's Olumiant". Fierce Pharma. Archived from the original on 8 July 2023. Retrieved 18 September 2023.
Further reading
- Guttman-Yassky E, Pavel AB, Diaz A, Zhang N, Del Duca E, Estrada Y, et al. (April 2022). "Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers". The Journal of Allergy and Clinical Immunology. 149 (4): 1318–1328. doi:10.1016/j.jaci.2021.10.036. PMID 34863853. S2CID 244824663.
- King B, Zhang X, Harcha WG, Szepietowski JC, Shapiro J, Lynde C, et al. (May 2023). "Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: a randomised, double-blind, multicentre, phase 2b-3 trial". Lancet. London, England. 401 (10387): 1518–1529. doi:10.1016/S0140-6736(23)00222-2. PMID 37062298. S2CID 258114404.
External links
- Clinical trial number NCT03732807 for "PF-06651600 for the Treatment of Alopecia Areata (ALLEGRO-2b/3)" at ClinicalTrials.gov
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.