Sex
| Part of a series on |
| Sex |
|---|
 |
| Biological terms |
|
| Sexual reproduction |
|
| Sexuality |
|
| Sexual system |
|
Sex is a trait that determines an individual's reproductive function, male or female, in animals and plants that propagate their species through sexual reproduction.[1][2] The type of gametes produced by an organism defines its sex. Commonly in plants and animals, male organisms produce smaller gametes (spermatozoa, sperm) while female organisms produce larger gametes (ova, often called egg cells).[3] Organisms that produce both types of gametes are called hermaphrodites.[2][4] During sexual reproduction, male and female gametes fuse to form zygotes that develop into offspring that inherit a selection of the traits of each parent.
Males and females of a species may be similar (sexual monomorphism), or have physical differences (sexual dimorphism). The differences reflect the different reproductive pressures the sexes experience. For instance, mate choice and sexual selection can accelerate the evolution of physical differences between the sexes.
The terms male and female typically do not apply in sexually undifferentiated species in which the individuals are isomorphic (look the same) and the gametes are isogamous (indistinguishable in size and shape), such as the green alga Ulva lactuca. If there are instead functional differences between gametes, such as in fungi,[5] they may be referred to as mating types.[6]
Sex is genetically determined in most mammals by the XY sex-determination system, where male mammals carry an X and a Y chromosome (XY), whereas female mammals carry two X chromosomes (XX). Other chromosomal sex-determination systems in animals include the ZW system in birds, and the X0 system in insects. Various environmental systems include temperature-dependent sex determination in reptiles and crustaceans.[7]
Reproduction
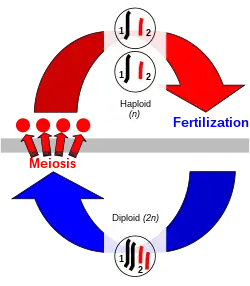
Sexual reproduction is production of offspring by the fusion of haploid gametes.[8][9] The codes for genetic traits are contained within the deoxyribonucleic acid (DNA) of chromosomes. By combining one set of chromosomes from each parent, an organism is formed containing a double set of chromosomes. This double-chromosome stage is called "diploid" while the single-chromosome stage is "haploid". Diploid organisms can, in turn, produce haploid cells (gametes) that randomly contain one of each of the chromosome pairs, via meiosis.[10] Meiosis also involves a stage of chromosomal crossover in which regions of DNA are exchanged between matched types of chromosomes, to form new pairs of mixed chromosomes, each of which is a blend of the genes of both parents.[10] This process is followed by a mitotic division, producing haploid gametes that contain one set of chromosomes. Crossing over to make new recombinant chromosomes and fertilization (the fusion of two gametes)[11] result in the new organism containing a different set of genetic traits from either parent.
Gametes may be externally similar (isogamy) or may differ in size and other aspects (anisogamy).[6] Oogamy is an extreme example of anisogamy, in which a large, non-motile gamete is fused with a smaller, usually motile one.[12] Isogamy is very common in unicellular organisms while anisogamy is common in multicellular organisms.[13] Individuals that exclusively produce large gametes are females, and those that exclusively produce small gametes are males.[14][3][15]
An individual that produces both types of gametes is a hermaphrodite.[4] Some hermaphrodites such as the roundworm Caenorhabditis elegans are able to self-fertilize and produce offspring on their own, without a second organism.[16] Some hermaphrodite animals such as Helix pomatia and Cepaea cannot self-fertilize.[17]
Some hermaphroditic plants are self-fertile, but plants have evolved multiple different mechanisms to avoid self-fertilization, involving sequential hermaphroditism (dichogamy), self-incompatibility or morphological mechanisms such as heterostyly (herkogamy).[18]: 73, 74
In the life-cycle of plants and multicellular algae, diploid and haploid multicellular phases alternate. The diploid organism is called the sporophyte because it produces haploid spores by meiosis, which, on germination, undergo mitotic cell division to produce multicellular haploid organisms, the gametophytes that produce gametes by mitosis.[19]
Animals

Sexually reproducing animals are diploid, and their single-celled gametes are the only haploid cells in their life cycles.[20] Animals have two gamete types: male spermatozoa (sperm) and female ova (egg cells).[21]
A spermatozoon, produced in vertebrates within the testes, is a small cell containing a single long flagellum which propels it.[22] Egg cells (ova) are produced within the ovaries. In oviparous species such as birds, the fertilized egg cell or zygote is provided with yolk, a nutrient supply which supports the development of the embryo.[23]
All animals that live outside of water use internal fertilization to transfer sperm directly into the female, thereby preventing the gametes from drying up.[24] Intromittent organs are the male copulation organs which help transport of sperm.[25]
Mammals
In mammals the female reproductive tract, called the vagina, connects with the uterus, an organ which directly supports the development of a fertilized embryo within, a process called gestation. In humans and other mammals the equivalent male organ is the penis, which enters the vagina to achieve insemination in a process called sexual intercourse. The penis contains a tube through which semen (a fluid containing sperm) travels. In Marsupials and placental mammals the fertilized egg develops within the female, receiving nutrition directly from its mother via a specialized organ called the placenta.[26]
Birds
In 97% of bird species, males do not have a penis.[25] Instead in most birds, both excretion and reproduction are done through a single posterior opening called the cloaca. Male and female birds touch cloacae to transfer sperm, a process called "cloacal kissing".[27]
Aquatic animals
Most aquatic animals such as fish and corals mate using external fertilization, where the eggs and sperm are released into, and combine within, the surrounding water.[28] However, some species like crustaceans use internal fertilization.[24] In seahorses, females use their ovipositors to deliver eggs into the males’ underside for fertilization and gestation. Pipefish and seahorses are the only species that entail male pregnancy.[29]
Insects
Most insects reproduce through oviparity, where a female mates with a male and the females lays the egg outside of her body.[30] A few groups of insects such as the Strepsiptera reproduce through traumatic insemination, where a male pierces a female's exoskeleton with his aedeagus.[31] In some harvester ants, a queen needs to mate with two types of males: one to reproduce queens and another to reproduce worker ants; these ants may be considered to have three or four sexes.[32]
Plants
In the green seaweed genus Ulva, there is no sexual specialization among the isomorphic individual plants, their sexual organs, or their isogamous gametes.[33] However, the majority of plants have specialized male and female gametes.[34][35]
The male gametes are the only cells in plants and green algae that have flagella. They are motile, able to swim to the egg cells of female gametophyte plants in films of water. Seed plants other than Cycads and Ginkgo have lost flagella entirely and are unable to swim in water. Once their pollen is delivered to the stigma of flowering plants, or the micropyle of gymnosperm ovules, their gametes are delivered to the egg cell by means of pollen tubes produced by one of the cells of the microgametophyte. Many plants, including conifers and grasses, are anemophilous producing lightweight pollen which is carried by wind to neighboring plants. Other plants, such as orchids,[36] have heavier, sticky pollen that is specialized for zoophily, transportation by animals. Plants attract insects such as bees or larger animals such as humming birds and bats with flowers containing rewards of nectar or resin.[37] These animals transport the pollen as they move to other flowers, which also contain female reproductive organs, resulting in cross-pollination.
Spermatophytes
In seed plants, male gametes are produced by extremely reduced multicellular microgametophytes known as pollen. The female gametes (egg cells) of seed plants are produced by larger megagametophytes contained within ovules. Once the egg cells are fertilized by male gametes produced by pollen, the ovules develop into seeds which contain the nutrients necessary for the initial development of the embryonic plant.[18]: 175
Conifers
In pines and other conifers, the sex organs are contained in the cones. The female cones (seed cones) produce seeds and male cones (pollen cones) produce pollen.[39] The female cones are longer lived and typically much larger and more durable. The ovules attached to the cone scales are not enclosed in an ovary, giving rise to the name gymnosperm meaning 'naked seed'. The smaller male cones produce pollen which is transported by wind to land in female cones. Naked seeds form after pollination, protected by the scales of the female cone.[40]
Angiosperms
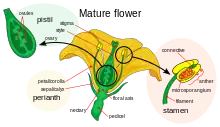
The sex organs of flowering plants are contained in flowers. The male parts of the flower are the stamens, which consist of the filaments supporting the anthers that produce the pollen.[41][42] The female parts in the flower, are the pistils, composed of one or more carpels. Carpels consist of an ovary, a style and a stigma. Within the ovary are ovules, which contain haploid megagametophytes that produce egg cells. When a pollen grain lands upon the stigma on top of a carpel's style, it germinates to produce a pollen tube that grows down through the tissues of the style into the carpel, where it delivers male gamete nuclei to fertilize the egg cell in an ovule that eventually develops into a seed. At the same time the ovary develops into a fruit.[43]
The majority of flowers are hermaphroditic (bisexual) and produce both male and female gametophytes in the same flowers. The male gametophytes form inside pollen grains and produce male gametes. The female gametophytes form inside ovules and produce female gametes.[44] Bisexual flowers that contain both male and female sexual organs are said to be perfect.[45][46]
Angiosperms may also have imperfect flowers, on the same or different plants, that lack one or other type of sex organs. Sometimes, as in the tree of heaven (Ailanthus altissima) and the European ash (Fraxinus excelsior) the panicles can produce different mixtures of functionally unisexual and functionally bisexual flowers on the same or different trees.[47]: 398, 615
Because flowering plants are immobile, they evolved flowers to attract animals such as insects to help in fertilization.[48]
Fungi

Most fungi are able to reproduce sexually and asexually and have both haploid and diploid stages in their life cycles.[5]: 214 Many fungi are isogamous, lacking male and female specialization.[49] Even fungi that are anisogamous are all hermaphroditic, which is why even anisogamous fungi are considered to be mating types rather than sexes.[50][51]: 182
Fungi may have complex allelic mating systems and many species of fungi have two mating types.[52] However, Coprinellus disseminatus has been estimated to have about 123 mating types, and in some species there are thousands of mating types.[49] For example, Schizophyllum commune has about 28,000 or more mating types.[53]
Some fungi, including that used as baker's yeast, have mating types that create a duality similar to male and female roles. Yeast with the same mating type do not fuse to form diploid cells, only with yeast carrying another mating type.[54]
Many species of higher fungi produce mushrooms as part of their sexual reproduction. Within the mushroom diploid cells are formed, later dividing into haploid spores.
Protozoa
Sexual reproduction is common among parasitic protozoa but rare among free-living protozoa, which usually reproduce asexually unless food is scarce or the environment changes drastically. Both anisogamy and isogamy are found in free-living protozoa.[55] Ciliates are all isogamous such as Tetrahymena thermophila, which has 7 mating types.[56]
Sexual systems
A sexual system is a distribution of male and female functions across organisms in a species.[57]
Animals
Approximately 95% of animal species are gonochoric (also known as dioecious) and about 5% are hermaphroditic.[57] This low percentage is due to the very large number of insect species, in which hermaphroditism is absent.[44] Hermaphroditism nevertheless occurs in 70% of animal phyla.[58]
Gonochoric individuals are either male or female throughout their lives.[59] Gonochorism is very common in vertebrates, about 99% of which gonochoric. The remaining 1% that are hermaphroditic are almost all fishes.[60] All birds and mammals are gonochoric.[61]
Plants
Roughly 5 to 6% of flowering plants are dioecious, resulting from between 871 and 5000 independent origins.[62] Consequently the majority are bisexual,[58] either hermaphrodite (with both stamens and pistil in the same flower) or monoecious (with separate male and female flowers on the same plant).[46][63] In dioecious species male and female sexes are on separate plants.[64] Dioecy is common in gymnosperms, in which 65% of species are dioecious, but most conifers are monoecious.[38]
Evolution of sex
A) anisogamy of motile cells, B) oogamy (egg cell and sperm cell), C) anisogamy of non-motile cells (egg cell and spermatia).
A) isogamy of motile cells, B) isogamy of non-motile cells, C) conjugation.
Sexual conflict underlies the evolutionary distinction between male and female with the distinction starting from anisogamy, the form of sexual reproduction that involves the union or fusion of two gametes that differ in size or form.[65] The evolution of anisogamy is also synonymous to the evolution of male and female sexes,[13] as well the starting point toward sexual dimorphism,[66] and lead to the evolution of many sex differences.[67]
It is generally accepted that anisogamy has evolved from isogamy[68] several times independently in different groups of eukaryotes, including protists, algae, plants and animals,[44] but its evolution has left no fossil evidence.[69] Until 2006 there was no genetic evidence for the evolutionary link between sexes and mating types due to plants and animals having no isogamous relatives.[70]
Anisogamy evolves due to disruptive selection leading to small gametes and large gametes.[71] In anisogamous species an intermediate gamete is unable to persist.[72] There should always be two gamete types, with all analyses showing that intermediate gamete sizes are eliminated due to selection.[73][74] As of 2016, it remains unclear if anisogamy first led to the evolution of gonochorism or the evolution of hermaphroditism.[58]: 213
Sex-determination systems
The biological cause of an organism developing into one sex or the other is called sex determination. The cause may be genetic, environmental, haplodiploidy, or multiple factors.[44] Within animals and other organisms that have genetic sex-determination systems, the determining factor may be the presence of a sex chromosome. In plants that are sexually dimorphic, such as the liverwort Marchantia polymorpha or the dioecious species in the flowering plant genus Silene, sex may also be determined by sex chromosomes.[75] Non-genetic systems may use environmental cues, such as the temperature during early development in crocodiles, to determine the sex of the offspring.[76]
Sex determination is often distinct from sex differentiation, sex determination is the designation for the development stage towards either male or female while sex differentiation is the pathway towards the development of the phenotype.[77]
Genetic
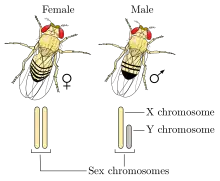
XY sex determination
Humans and most other mammals have an XY sex-determination system: the Y chromosome carries factors responsible for triggering male development, making XY sex determination mostly based on the presence or absence of the Y chromosome. It is the male gamete that determines the sex of the offspring.[78] In this system XX mammals typically are female and XY typically are male.[44] However, individuals with XXY or XYY are males, while individuals with X and XXX are females.[7]
XY sex determination is found in other organisms, including insects like the common fruit fly,[79] and some plants.[80] In some cases, it is the number of X chromosomes that determines sex rather than the presence of a Y chromosome.[7] In the fruit fly individuals with XY are male and individuals with XX are female; however, individuals with XXY or XXX can also be female, and individuals with X can be males.[81]
ZW sex determination
In birds, which have a ZW sex-determination system, the W chromosome carries factors responsible for female development, and default development is male.[82] In this case, ZZ individuals are male and ZW are female. It is the female gamete that determines the sex of the offspring. This system is used by birds, some fish, and some crustaceans.[7]
The majority of butterflies and moths also have a ZW sex-determination system. In groups like the Lepidoptera, females can have Z, ZZW, and even ZZWW.[83]
XO sex determination
In the X0 sex-determination system, males have one X chromosome (X0) while females have two (XX). All other chromosomes in these diploid organisms are paired, but organisms may inherit one or two X chromosomes. This system is found in most arachnids, insects such as silverfish (Apterygota), dragonflies (Paleoptera) and grasshoppers (Exopterygota), and some nematodes, crustaceans, and gastropods.[84][85]
In field crickets, for example, insects with a single X chromosome develop as male, while those with two develop as female.[86]
In the nematode Caenorhabditis elegans, most worms are self-fertilizing hermaphrodites with an XX karyotype, but occasional abnormalities in chromosome inheritance can give rise to individuals with only one X chromosome—these X0 individuals are fertile males (and half their offspring are male).[87]
ZO sex determination
In the Z0 sex-determination system, males have two Z chromosomes whereas females have one. This system is found in several species of moths.[88]
Environmental
For many species, sex is not determined by inherited traits, but instead by environmental factors such as temperature experienced during development or later in life.
The bonelliidae larvae can only develop as males when they encounter a female.[44]
In the fern Ceratopteris and other homosporous fern species, the default sex is hermaphrodite, but individuals which grow in soil that has previously supported hermaphrodites are influenced by the pheromone antheridiogen to develop as male.[89]
Sequential hermaphroditism

Some species can change sex over the course of their lifespan, a phenomenon called sequential hermaphroditism.[90] Teleost fishes are the only vertebrate lineage where sequential hermaphroditism occurs. In clownfish, smaller fish are male, and the dominant and largest fish in a group becomes female; when a dominant female is absent, then her partner changes sex. In many wrasses the opposite is true—the fish are initially female and become male when they reach a certain size.[91] Sequential hermaphroditism also occurs in plants such as Arisaema triphyllum.
Temperature-dependent sex determination
Many reptiles, including all crocodiles and most turtles, have temperature-dependent sex determination. In these species, the temperature experienced by the embryos during their development determines their sex.[44] In some turtles, for example, males are produced at lower temperatures than females; but Macroclemys females are produced at temperatures lower than 22 °C or above 28 °C, while males are produced in between those temperatures.[92]
Haplodiploidy
Other insects, including honey bees and ants, use a haplodiploid sex-determination system.[93] Diploid bees and ants are generally female, and haploid individuals (which develop from unfertilized eggs) are male. This sex-determination system results in highly biased sex ratios, as the sex of offspring is determined by fertilization (arrhenotoky or pseudo-arrhenotoky resulting in males) rather than the assortment of chromosomes during meiosis.[94]
Sex ratio
Most organisms which reproduce sexually have a 1:1 sex ratio of male and female births. The English statistician and biologist Ronald Fisher outlined why this is so in what has come to be known as Fisher's principle.[95] This essentially says the following:
- Suppose male births are less common than female.
- A newborn male then has better mating prospects than a newborn female, and therefore can expect to have more offspring.
- Therefore parents genetically disposed to produce males tend to have more than average numbers of grandchildren born to them.
- Therefore the genes for male-producing tendencies spread, and male births become more common.
- As the 1:1 sex ratio is approached, the advantage associated with producing males dies away.
- The same reasoning holds if females are substituted for males throughout. Therefore 1:1 is the equilibrium ratio.
Sex differences
Anisogamy is the fundamental difference between male and female.[96][97] Richard Dawkins has stated that it is possible to interpret all the differences between the sexes as stemming from this.[98]
Sex differences in humans include a generally larger size and more body hair in men, while women have larger breasts, wider hips, and a higher body fat percentage. In other species, there may be differences in coloration or other features, and may be so pronounced that the different sexes may be mistaken for two entirely different taxa.[99]
Sex differences in behavior
The sexes across gonochoric species usually differ in behavior. In most animal species females invest more in parental care,[100] although in some species, such as some coucals, the males invest more parental care.[101] Females also tend to be more choosy for who they mate with,[102] such as most bird species.[103] Males tend to be more competitive for mating than females.[13]
Sexual dimorphism

In many animals and some plants, individuals of male and female sex differ in size and appearance, a phenomenon called sexual dimorphism.[104] Sexual dimorphism in animals is often associated with sexual selection—the mating competition between individuals of one sex vis-à-vis the opposite sex.[99] In many cases, the male of a species is larger than the female. Mammal species with extreme sexual size dimorphism tend to have highly polygynous mating systems—presumably due to selection for success in competition with other males—such as the elephant seals. Other examples demonstrate that it is the preference of females that drives sexual dimorphism, such as in the case of the stalk-eyed fly.[105]
Females are the larger sex in a majority of animals.[104] For instance, female southern black widow spiders are typically twice as long as the males.[106] This size disparity may be associated with the cost of producing egg cells, which requires more nutrition than producing sperm: larger females are able to produce more eggs.[107][104]
Sexual dimorphism can be extreme, with males, such as some anglerfish, living parasitically on the female. Some plant species also exhibit dimorphism in which the females are significantly larger than the males, such as in the moss genus Dicranum[108] and the liverwort genus Sphaerocarpos.[109] There is some evidence that, in these genera, the dimorphism may be tied to a sex chromosome,[109][110] or to chemical signalling from females.[111]
In birds, males often have a more colourful appearance and may have features (like the long tail of male peacocks) that would seem to put them at a disadvantage (e.g. bright colors would seem to make a bird more visible to predators). One proposed explanation for this is the handicap principle.[112] This hypothesis argues that, by demonstrating he can survive with such handicaps, the male is advertising his genetic fitness to females—traits that will benefit daughters as well, who will not be encumbered with such handicaps.
Sexual monomorphism
Sexual monomorphism is when both sexes are similar in appearance and structure.[113] In primary sexual monomorphism both sexes have similar traits, and possibly similar genes, while in secondary sexual monomorphism both sexes have a trait that was historically in one sex.[114] In sexually monomorphic species both parents invest the same amount in their offspring and both sexes are choosy for whom to mate with.[115] Sexual monomorphism is also related to low levels of sexual selection.[116]
Monogamous species tend to be sexually monomorphic.[117] All pair bonded primates are sexually monomorphic,[118] including the tarsier family.[119] However, not all monogamous primates are sexually monomorphic,[118] there being only a few primate taxa where sexual monomorphism is associated with socially monogamous groups.[120]
Many bird species are sexually monomorphic.[121] Birds that are larger in size are more sexually monomorphic than smaller birds.[122]
In plants many sexually monomorphic species are hermaphrodites.[123]
Sex characteristics
Primary sex characteristics are organs directly involved in reproduction such as the testes or ovaries, while secondary sex characteristics in humans for example are body hair, breasts, and distribution of fat.[124]
Organisms that have intermediate sex characteristics between male and female are called intersex, this can be caused by extra sex chromosomes or by hormonal abnormality during fetal development.[125] The term intersex typically applies to abnormal members of gonochoric species rather than to hermaphroditic species.[126] Some species, such as the fruit fly (Drosophila melanogaster), and some crustaceans may have gynandromorphs.[127]
See also
- Sex and gender distinction
- Mating types
- Sex organ
- Sex allocation
- Sex assignment
- Sexing
References
- ↑ Stevenson A, Waite M (2011). Concise Oxford English Dictionary: Book & CD-ROM Set. OUP Oxford. p. 1302. ISBN 978-0-19-960110-3. Archived from the original on 11 March 2020. Retrieved 23 March 2018.
Sex: Either of the two main categories (male and female) into which humans and most other living things are divided on the basis of their reproductive functions. The fact of belonging to one of these categories. The group of all members of either sex.
- 1 2 Purves WK, Sadava DE, Orians GH, Heller HC (2000). Life: The Science of Biology. Macmillan. p. 736. ISBN 978-0-7167-3873-2. Archived from the original on 26 June 2019. Retrieved 23 March 2018.
A single body can function as both male and female. Sexual reproduction requires both male and female haploid gametes. In most species, these gametes are produced by individuals that are either male or female. Species that have male and female members are called dioecious (from the Greek for 'two houses'). In some species, a single individual may possess both female and male reproductive systems. Such species are called monoecious ("one house") or hermaphroditic.
- 1 2 Royle NJ, Smiseth PT, Kölliker M (9 August 2012). Kokko H, Jennions M (eds.). The Evolution of Parental Care. Oxford University Press. p. 103. ISBN 978-0-19-969257-6.
The answer is that there is an agreement by convention: individuals producing the smaller of the two gamete types-sperm or pollen- are males, and those producing larger gametes-eggs or ovules- are females.
- 1 2 Avise JC (18 March 2011). Hermaphroditism: A Primer on the Biology, Ecology, and Evolution of Dual Sexuality. Columbia University Press. pp. 1–7. ISBN 978-0-231-52715-6. Archived from the original on 11 October 2020. Retrieved 18 September 2020.
- 1 2 Moore D, Robson JD, Trinci AP (2020). 21st Century guidebook to fungi (2 ed.). Cambridge University press. pp. 211–228. ISBN 978-1-108-74568-0.
- 1 2 Kumar R, Meena M, Swapnil P (2019). "Anisogamy". In Vonk J, Shackelford T (eds.). Encyclopedia of Animal Cognition and Behavior. Cham: Springer International Publishing. pp. 1–5. doi:10.1007/978-3-319-47829-6_340-1. ISBN 978-3-319-47829-6. Archived from the original on 4 November 2020.
Anisogamy can be defined as a mode of sexual reproduction in which fusing gametes, formed by participating parents, are dissimilar in size.
- 1 2 3 4 Hake L, O'Connor C. "Genetic Mechanisms of Sex Determination | Learn Science at Scitable". www.nature.com. Retrieved 13 April 2021.
{{cite web}}: CS1 maint: url-status (link) - ↑ MacLean, Norman (1 January 2016). Dictionary of Genetics and Cell Biology. Macmillan International Higher Education. p. 362. ISBN 978-1-349-18905-2.
- ↑ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "The Benefits of Sex". Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN 978-0-8153-3218-3.
- 1 2 Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "Meiosis". Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN 978-0-8153-3218-3.
- ↑ Hine R, Martin E (2015). A Dictionary of Biology. Oxford University Press. p. 542. ISBN 978-0-19-871437-8.
- ↑ Allaby M (29 March 2012). A Dictionary of Plant Sciences. OUP Oxford. p. 350. ISBN 978-0-19-960057-1.
- 1 2 3 Lehtonen J, Kokko H, Parker GA (October 2016). "What do isogamous organisms teach us about sex and the two sexes?". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 371 (1706). doi:10.1098/rstb.2015.0532. PMC 5031617. PMID 27619696.
- ↑ Gee H (22 November 1999). "Size and the single sex cell". Nature. Retrieved 4 June 2018.
- ↑ Fusco G, Minelli A (10 October 2019). The Biology of Reproduction. Cambridge University Press. pp. 111–113, 297. ISBN 978-1-108-49985-9. Archived from the original on 1 April 2021. Retrieved 29 March 2021.
- ↑ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "Caenorhabditis Elegans: Development from the Perspective of the Individual Cell". Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN 978-0-8153-3218-3.
- ↑ Smith JM, Smith RT (24 August 1978). The Evolution of Sex. CUP Archive. p. 125. ISBN 978-0-521-29302-0.
- 1 2 Judd, Walter S.; Campbell, Christopher S.; Kellogg, Elizabeth A.; Stevens, Peter F.; Donoghue, Michael J. (2002). Plant systematics, a phylogenetic approach (2 ed.). Sunderland MA, USA: Sinauer Associates Inc. ISBN 0-87893-403-0.
- ↑ Gilbert SF (2000). "1.2. Multicellularity: Evolution of Differentiation". Developmental Biology (6th ed.). Sunderland (MA): Sinauer Associates. ISBN 978-0-87893-243-6.
- ↑ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "Mendelian genetics in eukaryotic life cycles". Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN 978-0-8153-3218-3.
- ↑ Judson, Olivia (14 August 2002). Dr. Tatiana's Sex Advice to All Creation: The Definitive Guide to the Evolutionary Biology of Sex. Henry Holt and Company. p. 88. ISBN 978-0-8050-6331-8.
- ↑ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "Sperm". Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN 978-0-8153-3218-3.
- ↑ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "Eggs". Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN 978-0-8153-3218-3.
- 1 2 Yoshida M, Asturiano JF (25 March 2020). Reproduction in Aquatic Animals: From Basic Biology to Aquaculture Technology. Springer Nature. p. 17. ISBN 978-981-15-2290-1.
- 1 2 Encyclopedia of Animal Behavior. Vol. 4. Academic Press. 21 January 2019. p. 505. ISBN 978-0-12-813252-4.
- ↑ Sadava DE, Heller HC, Purves WK, Orians GH, Hillis DM (2008). Life: The Science of Biology. W. H. Freeman. p. 905. ISBN 978-0-7167-7671-0.
- ↑ Ritchison G. "Avian Reproduction". people.eku.edu. Eastern Kentucky University. Archived from the original on 12 April 2008. Retrieved 3 April 2008.
- ↑ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "Fertilization". Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN 978-0-8153-3218-3.
- ↑ Jones AG, Avise JC (October 2003). "Male pregnancy". Current Biology. 13 (20): R791. doi:10.1016/j.cub.2003.09.045. PMID 14561416. S2CID 5282823.
- ↑ Klowden MJ (15 May 2013). Physiological Systems in Insects. Academic Press. p. 228. ISBN 978-0-12-415970-9.
- ↑ Peinert M, Wipfler B, Jetschke G, Kleinteich T, Gorb SN, Beutel RG, Pohl H (April 2016). "Traumatic insemination and female counter-adaptation in Strepsiptera (Insecta)". Scientific Reports. 6 (1): 25052. Bibcode:2016NatSR...625052P. doi:10.1038/srep25052. PMC 4850473. PMID 27125507.
- ↑ Schaffer A (27 September 2007). "Pas de Deux: Why Are There Only Two Sexes?". Slate. Archived from the original on 14 December 2007. Retrieved 30 November 2007.
- ↑ Smith GM (1947). "On the reproduction of some Pacific coast species of Ulva". American Journal of Botany. 34 (2): 80–87. doi:10.1002/j.1537-2197.1947.tb12961.x. JSTOR 2437232. PMID 20286318.
- ↑ Gilbert SF (2000). "Gamete Production in Angiosperms". Developmental Biology (6th ed.). Sunderland (MA): Sinauer Associates. ISBN 978-0-87893-243-6.
- ↑ Dusenbery DB (2009). Living at Micro Scale: The Unexpected Physics of Being Small. Harvard University Press. pp. 308–326. ISBN 978-0-674-03116-6.
- ↑ Micheneau C, Johnson SD, Fay MF (2009). "Orchid pollination: from Darwin to the present day". Botanical Journal of the Linnean Society. 161 (1): 1–19. doi:10.1111/j.1095-8339.2009.00995.x.
- ↑ Gustafsson M, Bittrich V (2008). "Evolution of morphological diversity and resin secretion in flowers of Clusia (Clusiaceae): insights from ITS sequence variation". Nordic Journal of Botany. 22 (2): 183–203. doi:10.1111/j.1756-1051.2002.tb01364.x.
- 1 2 Walas Ł, Mandryk W, Thomas PA, Tyrała-Wierucka Ż, Iszkuło G (2018). "Sexual systems in gymnosperms: A review" (PDF). Basic and Applied Ecology. 31: 1–9. doi:10.1016/j.baae.2018.05.009. S2CID 90740232.
- ↑ Farjon A (27 April 2010). A Handbook of the World's Conifers: Revised and Updated Edition. BRILL. p. 14. ISBN 978-90-474-3062-9.
- ↑ Farjon A (2008). The natural history of conifers. Portland, Oregon, US: Timber Press, Inc. pp. 16–21. ISBN 978-0-88192-869-3.
- ↑ Raven PH, Evert RF, Eichhorn SE (2005). Biology of Plants. Macmillan. pp. 436–451. ISBN 978-0-7167-1007-3.
- ↑ Avise J (18 March 2011). Hermaphroditism: A Primer on the Biology, Ecology, and Evolution of Dual Sexuality. Columbia University Press. pp. 43–46. ISBN 978-0-231-52715-6. Archived from the original on 11 October 2020. Retrieved 18 September 2020.
- ↑ Bell PR, Hemsley AR (2000). Green plants, their origin and diversity (2 ed.). Cambridge, UK: Cambridge University Press. p. 294. ISBN 0-521-64673-1.
- 1 2 3 4 5 6 7 Bachtrog D, Mank JE, Peichel CL, Kirkpatrick M, Otto SP, Ashman TL, et al. (July 2014). "Sex determination: why so many ways of doing it?". PLOS Biology. 12 (7): e1001899. doi:10.1371/journal.pbio.1001899. PMC 4077654. PMID 24983465.
- ↑ Renner SS, Ricklefs RE (1995). "Dioecy and its correlates in the flowering plants". American Journal of Botany. 82 (5): 596–606. doi:10.2307/2445418. JSTOR 2445418.
- 1 2 Sabath N, Goldberg EE, Glick L, Einhorn M, Ashman TL, Ming R, et al. (February 2016). "Dioecy does not consistently accelerate or slow lineage diversification across multiple genera of angiosperms". The New Phytologist. 209 (3): 1290–300. doi:10.1111/nph.13696. PMID 26467174.
- ↑ Stace CA (2019). New Flora of the British Isles (Fourth ed.). Middlewood Green, Suffolk, U.K.: C & M Floristics. ISBN 978-1-5272-2630-2.
- ↑ Raven PH, Evert RF, Eichhorn SE (2005). Biology of Plants. Macmillan. pp. 460–463. ISBN 978-0-7167-1007-3.
- 1 2 Heitman J, Howlett BJ, Crous PW, Stukenbrock EH, James TY, Gow NR (10 July 2020). Coelho M, Bakkeren G, Sun S, Hood M, Giraud T (eds.). The Fungal Kingdom. John Wiley & Sons. pp. 147–163. ISBN 978-1-55581-958-3.
- ↑ James T (1 December 2015). "Why mushrooms have evolved to be so promiscuous: Insights from evolutionary and ecological patterns". Fungal Biology Reviews. 29 (3–4): 167–178. doi:10.1016/j.fbr.2015.10.002. ISSN 1749-4613.
- ↑ Heitman, Joseph; Howlett, Barbara J.; Crous, Pedro W.; Stukenbrock, Eva H.; James, Timothy Yong; Gow, Neil A. R. (10 July 2020). The Fungal Kingdom. John Wiley & Sons. pp. 177–191. ISBN 978-1-55581-958-3.
- ↑ Watkinson SC, Boddy L, Money N (2015). The Fungi. Elsevier Science. p. 115. ISBN 978-0-12-382035-8. Archived from the original on 26 February 2020. Retrieved 18 February 2018.
- ↑ Lane N (13 October 2005). Power, Sex, Suicide: Mitochondria and the Meaning of Life. Oxford University Press, UK. p. 236. ISBN 978-0-19-280481-5.
- ↑ Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J (2000). "Section 14.1: Cell-Type Specification and Mating-Type Conversion in Yeast". Molecular Cell Biology (Fourth ed.). WH Freeman and Co. ISBN 978-0-7167-4366-8.
- ↑ Laybourn-Parry J (8 March 2013). A Functional Biology of Free-Living Protozoa. Springer Science & Business Media. pp. 86–88. ISBN 978-1-4684-7316-2.
- ↑ Wang G, Chen K, Zhang J, Deng S, Xiong J, He X, et al. (December 2020). "Drivers of Mating Type Composition in Tetrahymena thermophila". Genome Biology and Evolution. 12 (12): 2328–2343. doi:10.1093/gbe/evaa197. PMC 7846192. PMID 32946549.
- 1 2 Leonard, J. L. (22 August 2013). "Williams' Paradox and the Role of Phenotypic Plasticity in Sexual Systems". Integrative and Comparative Biology. 53 (4): 671–688. doi:10.1093/icb/ict088. ISSN 1540-7063. PMID 23970358.
- 1 2 3 Kliman, Richard (2016). Encyclopedia of Evolutionary Biology. Vol. 2. Academic Press. pp. 212–224. ISBN 978-0-12-800426-5. Archived from the original on 2016.
- ↑ West S (28 September 2009). Sex Allocation. Princeton University Press. pp. 1–2. ISBN 978-1-4008-3201-9.
- ↑ Kuwamura T, Sunobe T, Sakai Y, Kadota T, Sawada K (1 July 2020). "Hermaphroditism in fishes: an annotated list of species, phylogeny, and mating system". Ichthyological Research. 67 (3): 341–360. doi:10.1007/s10228-020-00754-6. ISSN 1616-3915. S2CID 218527927.
- ↑ Kobayashi K, Kitano T, Iwao Y, Kondo M (1 June 2018). Reproductive and Developmental Strategies: The Continuity of Life. Springer. p. 290. ISBN 978-4-431-56609-0.
- ↑ Renner, Susanne S. (2014). "The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database". American Journal of Botany. 101 (10): 1588–1596. doi:10.3732/ajb.1400196. PMID 25326608.
- ↑ Beentje H (2016). The Kew plant glossary (2 ed.). Royal Botanic Gardens, Kew: Kew Publishing. ISBN 978-1-84246-604-9.
- ↑ Leite Montalvão, Ana Paula; Kersten, Birgit; Fladung, Matthias; Müller, Niels Andreas (2021). "The Diversity and Dynamics of Sex Determination in Dioecious Plants". Frontiers in Plant Science. 11: 580488. doi:10.3389/fpls.2020.580488. ISSN 1664-462X. PMC 7843427. PMID 33519840.
- ↑ Gissis, Snait B.; Lamm, Ehud; Shavit, Ayelet (12 January 2018). Roughgarden, Joan (ed.). Landscapes of Collectivity in the Life Sciences. MIT Press. p. 228. ISBN 978-0-262-34266-7.
- ↑ Togashi, Tatsuya; Bartelt, John L.; Yoshimura, Jin; Tainaka, Kei-ichi; Cox, Paul Alan (21 August 2012). "Evolutionary trajectories explain the diversified evolution of isogamy and anisogamy in marine green algae". Proceedings of the National Academy of Sciences of the United States of America. 109 (34): 13692–13697. Bibcode:2012PNAS..10913692T. doi:10.1073/pnas.1203495109. ISSN 0027-8424. PMC 3427103. PMID 22869736.
- ↑ Székely, Tamás; Fairbairn, Daphne J.; Blanckenhorn, Wolf U. (5 July 2007). Sex, Size and Gender Roles: Evolutionary Studies of Sexual Size Dimorphism. OUP Oxford. pp. 167–169, 176, 185. ISBN 978-0-19-920878-4.
- ↑ Kumar, Awasthi & Ashok. Textbook of Algae. Vikas Publishing House. p. 363. ISBN 978-93-259-9022-7.
- ↑ Pitnick SS, Hosken DJ, Birkhead TR (21 November 2008). Sperm Biology: An Evolutionary Perspective. Academic Press. pp. 43–44. ISBN 978-0-08-091987-4.
- ↑ Sawada, Hitoshi; Inoue, Naokazu; Iwano, Megumi (7 February 2014). Sexual Reproduction in Animals and Plants. Springer. pp. 215–216. ISBN 978-4-431-54589-7.
- ↑ Low BS (4 January 2015). Why Sex Matters: A Darwinian Look at Human Behavior - Revised Edition. Princeton University Press. p. 32. ISBN 978-1-4008-5235-2.
This disruptive selection leads to anisogamy (unlike gametes) and a bimodal size distribution: small gametes (sperm) and large gametes (egg).
- ↑ Pitnick SS, Hosken DJ, Birkhead TR (21 November 2008). Sperm Biology: An Evolutionary Perspective. Academic Press. p. 60. ISBN 978-0-08-091987-4.
In anisogamous species, a third gamete size is either unable to invade or to persist, so two genders are evolutionarily stable
- ↑ Stearns, S. C. (21 November 2013). The Evolution of Sex and its Consequences. Birkhäuser. pp. 21, 81–82. ISBN 978-3-0348-6273-8.
- ↑ Lehtonen J, Parker GA (2014). "Gamete competition, gamete limitation, and the evolution of the two sexes". Molecular Human Reproduction. 20 (12): 1161–1168. doi:10.1093/molehr/gau068. PMID 25323972.
- ↑ Tanurdzic M, Banks JA (2004). "Sex-determining mechanisms in land plants". The Plant Cell. 16 Suppl: S61-71. doi:10.1105/tpc.016667. PMC 2643385. PMID 15084718.
- ↑ Warner DA, Shine R (January 2008). "The adaptive significance of temperature-dependent sex determination in a reptile". Nature. 451 (7178): 566–8. Bibcode:2008Natur.451..566W. doi:10.1038/nature06519. PMID 18204437. S2CID 967516.
- ↑ Beukeboom LW, Perrin N (2014). The Evolution of Sex Determination. Oxford University Press. p. 16. ISBN 978-0-19-965714-8.
- ↑ Wallis MC, Waters PD, Graves JA (October 2008). "Sex determination in mammals--before and after the evolution of SRY". Cellular and Molecular Life Sciences. 65 (20): 3182–95. doi:10.1007/s00018-008-8109-z. PMID 18581056. S2CID 31675679.
- ↑ Kaiser VB, Bachtrog D (2010). "Evolution of sex chromosomes in insects". Annual Review of Genetics. 44: 91–112. doi:10.1146/annurev-genet-102209-163600. PMC 4105922. PMID 21047257.
- ↑ Dellaporta SL, Calderon-Urrea A (October 1993). "Sex determination in flowering plants". The Plant Cell. 5 (10): 1241–51. doi:10.1105/tpc.5.10.1241. JSTOR 3869777. PMC 160357. PMID 8281039.
- ↑ Fusco G, Minelli A (10 October 2019). The Biology of Reproduction. Cambridge University Press. pp. 306–308. ISBN 978-1-108-49985-9.
- ↑ Smith CA, Katz M, Sinclair AH (February 2003). "DMRT1 is upregulated in the gonads during female-to-male sex reversal in ZW chicken embryos". Biology of Reproduction. 68 (2): 560–70. doi:10.1095/biolreprod.102.007294. PMID 12533420.
- ↑ Majerus ME (2003). Sex Wars: Genes, Bacteria, and Biased Sex Ratios. Princeton University Press. p. 59. ISBN 978-0-691-00981-0.
- ↑ Bull JJ (1983). Evolution of sex determining mechanisms. p. 17. ISBN 0-8053-0400-2.
- ↑ Thiriot-Quiévreux C (2003). "Advances in chromosomal studies of gastropod molluscs". Journal of Molluscan Studies. 69 (3): 187–202. doi:10.1093/mollus/69.3.187.
- ↑ Yoshimura A (2005). "Karyotypes of two American field crickets: Gryllus rubens and Gryllus sp. (Orthoptera: Gryllidae)". Entomological Science. 8 (3): 219–222. doi:10.1111/j.1479-8298.2005.00118.x. S2CID 84908090.
- ↑ Meyer BJ (1997). "Sex Determination and X Chromosome Dosage Compensation: Sexual Dimorphism". In Riddle DL, Blumenthal T, Meyer BJ, Priess JR (eds.). C. elegans II. Cold Spring Harbor Laboratory Press. ISBN 978-0-87969-532-3.
- ↑ Handbuch Der Zoologie / Handbook of Zoology. Walter de Gruyter. 1925. ISBN 978-3-11-016210-3. Archived from the original on 11 October 2020. Retrieved 29 September 2020 – via Google Books.
- ↑ Tanurdzic M, Banks JA (2004). "Sex-determining mechanisms in land plants". The Plant Cell. 16 Suppl (Suppl): S61-71. doi:10.1105/tpc.016667. PMC 2643385. PMID 15084718.
- ↑ Fusco G, Minelli A (10 October 2019). The Biology of Reproduction. Cambridge University Press. p. 124. ISBN 978-1-108-49985-9.
- ↑ Todd EV, Liu H, Muncaster S, Gemmell NJ (2016). "Bending Genders: The Biology of Natural Sex Change in Fish". Sexual Development. 10 (5–6): 223–241. doi:10.1159/000449297. PMID 27820936. S2CID 41652893.
- ↑ Gilbert SF (2000). "Environmental Sex Determination". Developmental Biology. 6th Edition.
- ↑ Charlesworth B (August 2003). "Sex determination in the honeybee". Cell. 114 (4): 397–8. doi:10.1016/S0092-8674(03)00610-X. PMID 12941267.
- ↑ de la Filia A, Bain S, Ross L (June 2015). "Haplodiploidy and the reproductive ecology of Arthropods" (PDF). Current Opinion in Insect Science. 9: 36–43. doi:10.1016/j.cois.2015.04.018. hdl:20.500.11820/b540f12f-846d-4a5a-9120-7b2c45615be6. PMID 32846706.
- ↑ Hamilton, W.D. (1967). "Extraordinary sex ratios". Science. 156 (3774): 477–488. Bibcode:1967Sci...156..477H. doi:10.1126/science.156.3774.477. PMID 6021675.
- ↑ Whitfield J (June 2004). "Everything you always wanted to know about sexes". PLOS Biology. 2 (6): e183. doi:10.1371/journal.pbio.0020183. PMC 423151. PMID 15208728.
One thing biologists do agree on is that males and females count as different sexes. And they also agree that the main difference between the two is gamete size: males make lots of small gametes—sperm in animals, pollen in plants—and females produce a few big eggs.
- ↑ Pierce BA (2012). Genetics: A Conceptual Approach. W. H. Freeman. p. 74. ISBN 978-1-4292-3252-4.
- ↑ Dawkins, Richard (2016). The Selfish Gene. Oxford University Press. pp. 183–184. ISBN 978-0-19-878860-7.
However, there is one fundamental feature of the sexes which can be used to label males as males, and females as females, throughout animals and plants. This is that the sex cells or ‘gametes' of males are much smaller and more numerous than the gametes of females. This is true whether we are dealing with animals or plants. One group of individuals has large sex cells, and it is convenient to use the word female for them. The other group, which it is convenient to call male, has small sex cells. The difference is especially pronounced in reptiles and in birds, where a single egg cell is big enough and nutritious enough to feed a developing baby for. Even in humans, where the egg is microscopic, it is still many times larger than the sperm. As we shall see, it is possible to interpret all the other differences between the sexes as stemming from this one basic difference.
- 1 2 Mori, Emiliano; Mazza, Giuseppe; Lovari, Sandro (2017). "Sexual Dimorphism". In Vonk, Jennifer; Shackelford, Todd (eds.). Encyclopedia of Animal Cognition and Behavior. Cham: Springer International Publishing. pp. 1–7. doi:10.1007/978-3-319-47829-6_433-1. ISBN 978-3-319-47829-6. Retrieved 5 June 2021.
- ↑ Kliman, Richard (14 April 2016). Herridge, Elizabeth J; Murray, Rosalind L; Gwynne, Darryl T; Bussiere, Luc (eds.). Encyclopedia of Evolutionary Biology. Vol. 2. Academic Press. pp. 453–454. ISBN 978-0-12-800426-5.
- ↑ Henshaw, Jonathan M.; Fromhage, Lutz; Jones, Adam G. (28 August 2019). "Sex roles and the evolution of parental care specialization". Proceedings of the Royal Society B: Biological Sciences. 286 (1909): 20191312. doi:10.1098/rspb.2019.1312. PMC 6732396. PMID 31455191.
- ↑ "Sexual Selection | Learn Science at Scitable". www.nature.com. Retrieved 25 July 2021.
- ↑ Reboreda, Juan Carlos; Fiorini, Vanina Dafne; Tuero, Diego Tomás (24 April 2019). Behavioral Ecology of Neotropical Birds. Springer. p. 75. ISBN 978-3-030-14280-3.
- 1 2 3 Choe J (21 January 2019). "Body Size and Sexual Dimorphism". In Cox R (ed.). Encyclopedia of Animal Behavior. Vol. 2. Academic Press. pp. 7–11. ISBN 978-0-12-813252-4.
- ↑ Wilkinson GS, Reillo PR (22 January 1994). "Female choice response to artificial selection on an exaggerated male trait in a stalk-eyed fly" (PDF). Proceedings of the Royal Society B. 225 (1342): 1–6. Bibcode:1994RSPSB.255....1W. CiteSeerX 10.1.1.574.2822. doi:10.1098/rspb.1994.0001. S2CID 5769457. Archived from the original (PDF) on 10 September 2006.
- ↑ Drees BM, Jackman J (1999). "Southern black widow spider". Field Guide to Texas Insects. Houston, Texas: Gulf Publishing Company. Archived from the original on 31 August 2003. Retrieved 8 August 2012 – via Extension Entomology, Insects.tamu.edu, Texas A&M University.
- ↑ Stuart-Smith J, Swain R, Stuart-Smith R, Wapstra E (2007). "Is fecundity the ultimate cause of female-biased size dimorphism in a dragon lizard?". Journal of Zoology. 273 (3): 266–272. doi:10.1111/j.1469-7998.2007.00324.x.
- ↑ Shaw AJ (2000). "Population ecology, population genetics, and microevolution". In Shaw AJ, Goffinet B (eds.). Bryophyte Biology. Cambridge: Cambridge University Press. pp. 379–380. ISBN 978-0-521-66097-6.
- 1 2 Schuster RM (1984). "Comparative Anatomy and Morphology of the Hepaticae". New Manual of Bryology. Vol. 2. Nichinan, Miyazaki, Japan: The Hattori botanical Laboratory. p. 891.
- ↑ Crum HA, Anderson LE (1980). Mosses of Eastern North America. Vol. 1. New York: Columbia University Press. p. 196. ISBN 978-0-231-04516-2.
- ↑ Briggs DA (1965). "Experimental taxonomy of some British species of genus Dicranum". New Phytologist. 64 (3): 366–386. doi:10.1111/j.1469-8137.1965.tb07546.x.
- ↑ Zahavi A, Zahavi A (1997). The handicap principle: a missing piece of Darwin's puzzle. Oxford University Press. ISBN 978-0-19-510035-8.
- ↑ Jones, Richard E.; Lopez, Kristin H. (28 September 2013). Human Reproductive Biology. Academic Press. p. 364. ISBN 978-0-12-382185-0.
- ↑ West-Eberhard, Mary Jane (10 April 2003). Developmental Plasticity and Evolution. OUP USA. p. 262. ISBN 978-0-19-512235-0.
- ↑ Phelan, Jay (2 March 2009). What Is Life?: A Guide to Biology w/Prep-U. Macmillan. p. 359. ISBN 978-1-4292-8079-2.
- ↑ Motta, Philip J. (6 December 2012). The butterflyfishes: success on the coral reef. Springer Science & Business Media. p. 66. ISBN 978-94-009-2325-6.
- ↑ Reichard, Ulrich H.; Boesch, Christophe (11 September 2003). Monogamy: Mating Strategies and Partnerships in Birds, Humans and Other Mammals. Cambridge University Press. p. 125. ISBN 978-0-521-52577-0.
- 1 2 Strier, Karen B. (29 July 2021). Primate Behavioral Ecology. Routledge. ISBN 978-1-000-35921-3.
- ↑ Setchell, Joanna M. (26 September 2019). Studying Primates: How to Design, Conduct and Report Primatological Research. Cambridge University Press. p. 113. ISBN 978-1-108-42171-3.
- ↑ Strier, Karen B. (8 January 2016). Primate Ethnographies. Routledge. ISBN 978-1-317-34516-9.
- ↑ Sutherland, William J.; Newton, Ian; Green, Rhys E. (17 June 2004). Bird Ecology and Conservation: A Handbook of Techniques. OUP Oxford. p. 227. ISBN 978-0-19-852086-3.
- ↑ Johnston, Richard F.; Janiga, Marián (1995). Feral Pigeons. Oxford University Press. p. 21. ISBN 978-0-19-508409-2.
- ↑ Jain, Shri Mohan; Al-Khayri, Jameel M.; Johnson, Dennis V. (13 August 2011). Date Palm Biotechnology. Springer Science & Business Media. p. 552. ISBN 978-94-007-1318-5.
- ↑ Pack PE (20 December 2016). CliffsNotes AP Biology, 5th Edition. Houghton Mifflin Harcourt. p. 219. ISBN 978-0-544-78417-8.
- ↑ "intersex | Definition & Facts". Encyclopedia Britannica. Archived from the original on 25 July 2020. Retrieved 11 October 2020.
The [intersex] condition usually results from extra chromosomes or a hormonal abnormality during embryological development.
- ↑ Farrell A (1 June 2011). Encyclopedia of Fish Physiology: From Genome to Environment. Academic Press. ISBN 978-0-08-092323-9.
Thus, strictly speaking, all hermaphrodites are intersex at one time point, but not all intersexes are hermaphrodites. This definition is usually applied to gonochoristic species to describe those individuals that are not normal for the species.
- ↑ Subramoniam, Thanumalaya (27 September 2016). Sexual Biology and Reproduction in Crustaceans. Academic Press. p. 95. ISBN 978-0-12-809606-2.
Further reading
- Arnqvist G, Rowe L (2005). Sexual conflict. Princeton University Press. ISBN 978-0-691-12217-5.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). Molecular Biology of the Cell (4th ed.). New York: Garland Science. ISBN 978-0-8153-3218-3.
- Ellis H (1933). Psychology of Sex. London: W. Heinemann Medical Books. N.B.: One of many books by this pioneering authority on aspects of human sexuality.
- Gilbert SF (2000). Developmental Biology (6th ed.). Sinauer Associates, Inc. ISBN 978-0-87893-243-6.
- Maynard-Smith J (1978). The Evolution of Sex. Cambridge University Press. ISBN 978-0-521-29302-0.
External links
- Human Sexual Differentiation Archived 9 February 2010 at the Wayback Machine by P. C. Sizonenko