Sodium nitrite (medical use)
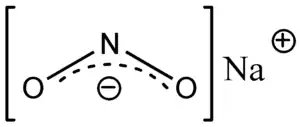 Chemical structure | |
| Clinical data | |
|---|---|
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Defined daily dose | not established[1] |
| External links | |
| AHFS/Drugs.com | FDA Professional Drug Information |
| Chemical and physical data | |
| Formula | NaNO2 |
| Molar mass | 68.9953 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Sodium nitrite is used as a medication together with sodium thiosulfate to treat cyanide poisoning.[2] It is only recommended in severe cases of cyanide poisoning.[3] In those who have both cyanide poisoning and carbon monoxide poisoning sodium thiosulfate by itself is usually recommended.[4] It is given by slow injection into a vein.[2]
Side effects can include low blood pressure, headache, shortness of breath, loss of consciousness, and vomiting.[2] Greater care should be taken in people with underlying heart disease.[2] People's levels of methemoglobin should be regularly checked during treatment.[2] While not well studied during pregnancy, there is some evidence of potential harm to the baby.[5] Sodium nitrite is believed to work by creating methemoglobin that then binds with cyanide and thus removes it from the mitochondria.[5]
Sodium nitrite came into medical use in the 1920s and 1930s.[6][7] It is on the World Health Organization's List of Essential Medicines.[8] The cost in the United States together with sodium thiosulfate is about US$110.[9]
Dosage
The defined daily dose is not established[1]
References
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 22 January 2021. Retrieved 13 September 2020.
- 1 2 3 4 5 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 65. hdl:10665/44053. ISBN 9789241547659.
- ↑ "Sodium Nitrite Solution for Injection - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. Archived from the original on 18 September 2017. Retrieved 15 January 2017.
- ↑ Baren, Jill M. (2008). Pediatric Emergency Medicine. Elsevier Health Sciences. p. 1018. ISBN 978-1416000877. Archived from the original on 2017-01-16.
- 1 2 "Sodium Nitrite Injection - FDA prescribing information, side effects and uses". www.drugs.com. Archived from the original on 2017-01-18.
- ↑ Dart, Richard C. (2004). Medical Toxicology. Lippincott Williams & Wilkins. p. 172. ISBN 9780781728454. Archived from the original on 2017-01-16.
- ↑ Bryan, Nathan S.; Loscalzo, Joseph (2011). Nitrite and Nitrate in Human Health and Disease. Springer Science & Business Media. p. 226. ISBN 9781607616160. Archived from the original on 2017-01-16.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Gasco, L; Rosbolt, MB; Bebarta, VS (April 2013). "Insufficient stocking of cyanide antidotes in US hospitals that provide emergency care". Journal of Pharmacology & Pharmacotherapeutics. 4 (2): 95–102. doi:10.4103/0976-500x.110875. PMC 3669589. PMID 23761707.
External links
| Identifiers: |
|---|