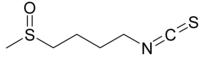
Sulforaphane
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-Isothiocyanato-4-(methanesulfinyl)butane | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C6H11NOS2 |
| Molar mass | 177.29 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sulforaphane (sometimes sulphoraphane in British English) is a compound within the isothiocyanate group of organosulfur compounds.[1] It is obtained from cruciferous vegetables such as broccoli, Brussels sprouts, and cabbages. It is produced when the enzyme myrosinase transforms glucoraphanin, a glucosinolate, into sulforaphane upon damage to the plant (such as from chewing or boiling during food preparation), which allows the two compounds to mix and react. Young sprouts of broccoli and cauliflower are particularly rich in glucoraphanin.[1]
 Glucoraphanin, the glucosinolate precursor to sulforaphane |
Occurrence and isolation
Sulforaphane occurs in broccoli sprouts, which, among cruciferous vegetables, have the highest concentration of glucoraphanin, the precursor to sulforaphane.[1][2] It is also found in cabbage, cauliflower, Brussels sprouts, bok choy, kale, collards, mustard greens, and watercress.[1]
Research
Although there has been some basic research on how sulforaphane might exert beneficial effects in vivo, there is no high-quality evidence for its efficacy against human diseases.[1][3]
See also
- Raphanin
References
- 1 2 3 4 5 "Isothiocyanates". Linus Pauling Institute. Micronutrient Information Center, Linus Pauling Institute, Oregon State University. March 2017. Retrieved 19 November 2018.
- ↑ Houghton, C. A.; Fassett, R. G.; Coombes, J. S. (2013). "Sulforaphane: Translational research from laboratory bench to clinic". Nutrition Reviews. 71 (11): 709–26. doi:10.1111/nure.12060. PMID 24147970.
- ↑ van Die, MD; Bone, KM; Emery, J; Williams, SG; Pirotta, MV; Paller, CJ (April 2016). "Phytotherapeutic interventions in the management of biochemically recurrent prostate cancer: a systematic review of randomised trials". BJU Int. 117 (S4): 17–34. doi:10.1111/bju.13361. PMC 8631186. PMID 26898239.