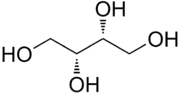
Threitol
 | |
| Names | |
|---|---|
| Systematic IUPAC name
(2R,3R)-Butane-1,2,3,4-tetrol | |
| Other names
(2R,3R)-Butane-1,2,3,4-tetraol (not recommended) | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
Beilstein Reference |
1719752 |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.150.149 |
| EC Number |
|
Gmelin Reference |
1782960 |
| KEGG | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C4H10O4 |
| Molar mass | 122.12 |
| Appearance | Solid |
| Melting point | 88 to 90 °C (190 to 194 °F; 361 to 363 K) |
| Hazards | |
| GHS labelling:[2] | |
Pictograms |
 |
Signal word |
Warning |
Hazard statements |
H315, H319, H335 |
Precautionary statements |
P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Threitol is a four-carbon sugar alcohol with the molecular formula C4H10O4. It is primarily used as an intermediate in the chemical synthesis of other compounds. It is the diastereomer of erythritol.
In living things, threitol is found in the edible fungus Armillaria mellea.[3] It serves as a cryoprotectant (antifreeze agent) in the Alaskan beetle Upis ceramboides.[4]
See also
- Antifreeze protein
- Dithiothreitol, a thiol derivative of threitol
References
- ↑ Threitol at Sigma-Alrich
- ↑ "D-Threitol". pubchem.ncbi.nlm.nih.gov. Retrieved 12 December 2021.
- ↑ Elks, J.; Ganellin, C. R. (1990). Dictionary of Drugs. doi:10.1007/978-1-4757-2085-3. ISBN 978-1-4757-2087-7.
- ↑ Walters, K. R. Jr; Pan, Q.; Serianni, A. S.; Duman, J. G. (2009). "Cryoprotectant biosynthesis and the selective accumulation of threitol in the freeze-tolerant Alaskan beetle, Upis ceramboides". Journal of Biological Chemistry. 284 (25): 16822–16831. doi:10.1074/jbc.M109.013870. PMC 2719318. PMID 19403530.
External links
 Media related to Threitol at Wikimedia Commons
Media related to Threitol at Wikimedia Commons
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.