Uridine triacetate
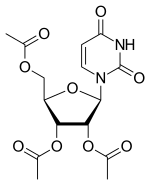 | |
| Names | |
|---|---|
| Trade names | Vistogard, Xuriden |
| Other names | Vistonuridine |
IUPAC name
| |
| Clinical data | |
| Main uses | Hereditary orotic aciduria, poisoning due to fluorouracil or capecitabine[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Onset of action | Tmax = 2–3 hours |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a616020 |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Metabolism | Pyrimidine catabolic pathway |
| Elimination half-life | 2–2.5 hours |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C15H18N2O9 |
| Molar mass | 370.314 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Uridine triacetate, formerly known as vistonuridine, is a medication used to treat hereditary orotic aciduria and poisoning due to fluorouracil or capecitabine.[1] For poisoning it should be used within 4 days and in an overdose may be used regardless of the presence of symptoms.[1] It is taken by mouth.[1][2]
Side effects may include nausea or diarrhea.[1] Use in pregnancy and breastfeeding is of unclear safety.[3] It is a prodrug of uridine; by which it acts.[2][1]
Uridine triacetate was approved for medical use in the United States in 2015.[1] It is not approved in Europe.[4] In the United States it costs about 4,200 USD per 10 gram dose as of 2021.[5] In the United States it is only available through specialty pharmacies.[1] In Canada approval is required from the Health Canada Special Access Programme.[6] Brand names include Vistogard and Xuriden.[1]
Medical uses
Dosage
For poisoning a dose of 10 gm four times per day for 5 days is typically used.[1]
For hereditary orotic aciduria a dose of 60 mg/kg per day to a maximum of 8 gm per day is typically used.[1]
Society and culture
Names
Uridine triacetate is the INN.[7] Brand names include Xuriden /ˈzʊərədɛn/ ZOOR-ə-den);[8] and Vistogard.[9][10][11]
Cost
The cost of the medication used to treat an overdose is about 84,000 USD in the United States as of 2021.[5]
References
- 1 2 3 4 5 6 7 8 9 10 11 "Uridine Triacetate Monograph for Professionals". Drugs.com. Archived from the original on 8 December 2019. Retrieved 13 September 2021.
- 1 2 Saif, Muhammad Wasif (4 March 2019). "Uridine triacetate - an antidote in the treatment of 5-fluorouracil or capecitabine poisoning". Expert Opinion on Orphan Drugs. 7 (3): 95–103. doi:10.1080/21678707.2019.1591273.
- ↑ "Uridine Use During Pregnancy". Drugs.com. Archived from the original on 30 October 2020. Retrieved 13 September 2021.
- ↑ "FDA approves drug for genetic condition that affects 20 patients worldwide". The Pharmaceutical Journal. 2015. doi:10.1211/PJ.2015.20069307.
- 1 2 "Vistogard Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 22 January 2021. Retrieved 13 September 2021.
- ↑ "BC Cancer Agency Management Guidelines Management of 5-fluorouracil (5FU) infusion overdose" (PDF). Archived (PDF) from the original on 8 February 2020. Retrieved 13 September 2021.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 65" (PDF). World Health Organization. p. 92. Archived (PDF) from the original on 18 May 2016. Retrieved 12 March 2017.
- ↑ "Xuriden- uridine triacetate granule". DailyMed. 16 December 2019. Archived from the original on 23 October 2020. Retrieved 20 October 2020.
- ↑ "Vistogard- uridine triacetate granule". DailyMed. 15 November 2018. Archived from the original on 23 October 2020. Retrieved 20 October 2020.
- ↑ "BTG Announces FDA Approval of Vistogard (Uridine Triacetate) as Antidote to Overdose and Early Onset, Severe, or Life-Threatening Toxicities from Chemotherapy Drugs 5-Fluorouracil (5-FU) or Capecitabine". BTG International Ltd. 11 December 2015. Archived from the original on 8 April 2017. Retrieved 12 March 2017.
- ↑ "Approved Drugs — Uridine Triacetate". U.S. Food and Drug Administration. Archived from the original on 13 March 2017. Retrieved 12 March 2017.
External links
| Identifiers: |
|---|
- "Uridine triacetate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 8 December 2019. Retrieved 24 January 2021.