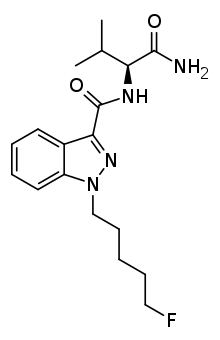
5F-AB-PINACA
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H25FN4O2 |
| Molar mass | 348.422 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
5F-AB-PINACA is an indazole-based synthetic cannabinoid that is derived from a series of compounds originally developed by Pfizer in 2009 as an analgesic medication, and has been sold online as a designer drug.[1][2]
5F-AB-PINACA has been reported to be a potent agonist of the CB1 receptor and CB2 receptor with EC50 values of 0.48 nM and 2.6 nM respectively.[3] Its metabolism has been described in literature.[4][5]
Legality
China
As of October 2015 5F-AB-PINACA is a controlled substance in China.[6]
Germany
5F-AB-PINACA is an Anlage II controlled substance in Germany as of May 2015.[7]
Singapore
It is also controlled under the Fifth Schedule of the Misuse of Drugs Act (MDA) in Singapore as of May 2015.[8]
See also
References
- ↑ WO 2009106980, Buchler I, Hayes M, Hegde S, Hockerman S, Jones D, Kortum S, Rico J, Tenbrink R, Wu KK, "Indazole derivatives", published 2009-09-03, assigned to Pfizer
- ↑ "5F-AB-PINACA". Cayman Chemical. Retrieved 5 July 2015.
- ↑ Banister SD, Moir M, Stuart J, Kevin RC, Wood KE, Longworth M, et al. (September 2015). "Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA". ACS Chemical Neuroscience. 6 (9): 1546–59. doi:10.1021/acschemneuro.5b00112. PMID 26134475.
- ↑ Wohlfarth A, Castaneto MS, Zhu M, Pang S, Scheidweiler KB, Kronstrand R, Huestis MA (May 2015). "Pentylindole/Pentylindazole Synthetic Cannabinoids and Their 5-Fluoro Analogs Produce Different Primary Metabolites: Metabolite Profiling for AB-PINACA and 5F-AB-PINACA". The AAPS Journal. 17 (3): 660–77. doi:10.1208/s12248-015-9721-0. PMC 4406957. PMID 25721194.
- ↑ Jang M, Shin I, Kim J, Yang W (July 2015). "Simultaneous quantification of 37 synthetic cannabinoid metabolites in human urine by liquid chromatography-tandem mass spectrometry". Forensic Toxicology. 33 (2): 221–234. doi:10.1007/s11419-015-0265-x. S2CID 3038555.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.
- ↑ "Gesetz über den Verkehr mit Betäubungsmitteln (Betäubungsmittelgesetz - BtMG) Anlage II (zu § 1 Abs. 1) (verkehrsfähige, aber nicht verschreibungsfähige Betäubungsmittel)" (in German). Retrieved 5 July 2015.
- ↑ "CNB NEWS RELEASE". Central Narcotics Bureau (CNB). 30 April 2015. Archived from the original on 15 July 2015. Retrieved 24 July 2015.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.