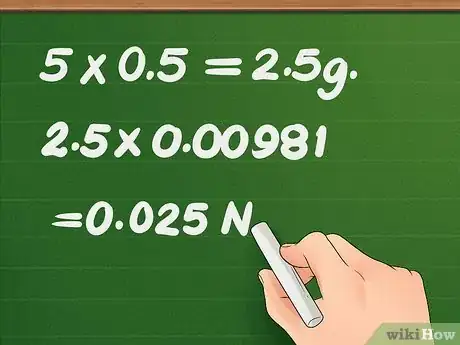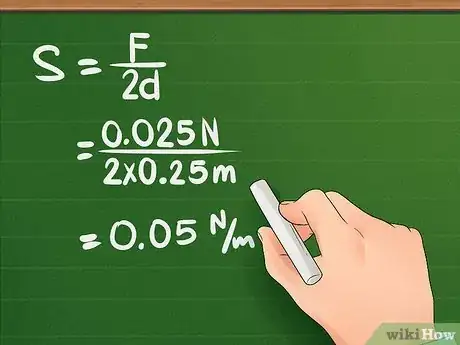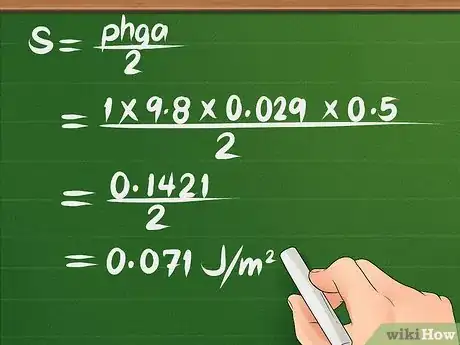This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
There are 11 references cited in this article, which can be found at the bottom of the page.
wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, 85% of readers who voted found the article helpful, earning it our reader-approved status.
This article has been viewed 138,176 times.
Surface tension refers to the ability of a liquid to resist the force of gravity. For example, water forms droplets on a table because the water molecules at the surface group together against the force of gravity.[1] Surface tension is what allows a denser object, such as an insect, to be able to float on the water's surface. Surface tension is measured by the amount of force (N) exerted on a unit such as length (m) or the amount of energy of a measured area. These are measured as Newton per meter (or N/meter).[2] The forces that water molecules exert on each other, or cohesive forces, cause the tension and are responsible for the shape of water (or other liquid) drops. You can measure surface tension with a few household items and a calculator.
Steps
Measuring Surface Tension with a Balance Beam
-
1Define the equation to solve for surface tension. In this experiment, the equation for surface tension will be determined by the equation F = 2sd. F is the force in newtons (N), s is the surface tension in (N/m), and d is the length of the needle used in the experiment. Rearranging the equation to solve for surface tension yields s = F/2d.[3]
- The force will be calculated at the end of the experiment.
- Measure the length of the needle in meters using a ruler before starting the experiment.
-
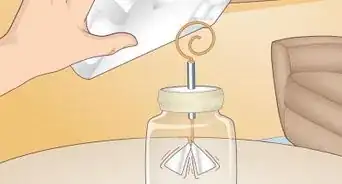
2Construct a small balance beam.[4] In this experiment, you will use a balance beam and a small needle floating on the surface of water to measure surface tension. The balance beam needs to be well constructed so that you can get an accurate result. You can use many different types of materials for this, just make sure the center beam is something sturdy like wood, plastic, or dense cardboard.
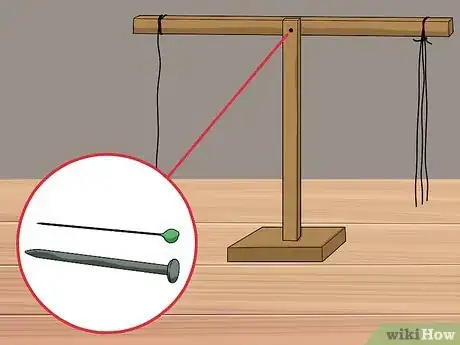
- Mark the center of the material to be used for your beam (straw, plastic ruler) and drill or poke a hole through it; this will be the fulcrum point (the point that allows the beam to rotate freely). If you are using a plastic straw you can just poke a pin or nail right through it.
- Drill or poke a hole at each end of the beam ensuring that they are the same distance from the middle. Thread a string through each hole to serve as holders for the balance dishes. Make sure that there is 1 string for each hole at either end.
- Rest the nail horizontally between two stacks of books so that the center beam can rotate freely.
Advertisement -
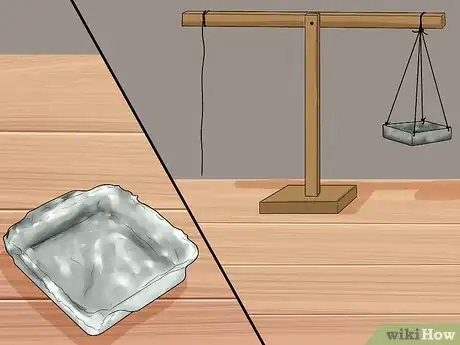
3Fold a piece of aluminum foil to form a box or dish. The dish does not have to be exactly square or round. The dish will be filled with water or another weight, so make sure it is sturdy enough to support this.
- Hang the box or dish from one end of the beam. Poke small holes in the sides of the dish and thread the string through to hold up the dish.
-
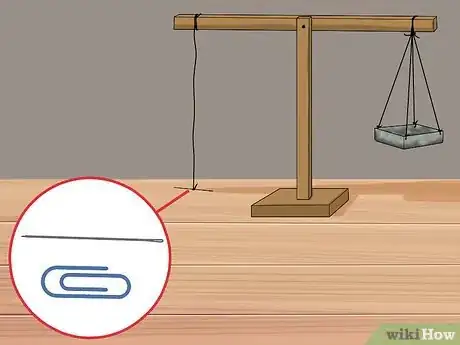
4Hang a needle or paper clip horizontally from the other end of the beam with thread. On the opposite side of the beam, tie a paper clip or needle to the end of the string so that it lays flat. For the experiment to work, it is important that the paper clip or needle is horizontal.
-
5Place a piece of material such as clay or play-doh on the beam to counterbalance the aluminum container. Before beginning the experiment, you want to make sure that the beam is lying flat. The dish will be heavier than the needle, causing the beam to lower in the direction of the dish. Add enough clay to the opposite side of the beam so that the beam is level.
- This is called counterbalancing. The clay does not affect the calculations because it is balancing out the beam.
-
6Place the needle or paper clip hanging from the beam into a container of water. This step may require some extra effort to ensure the needle is resting just on the top of the water’s surface. You do not want the needle submerged in the water. Fill a container with water (or other liquid of unknown surface tension) and place it underneath the needle at a height that allows the needle to rest directly on top the surface.
- Make sure the string holding the needle in place remains taut once the needle is on top of the water.
-
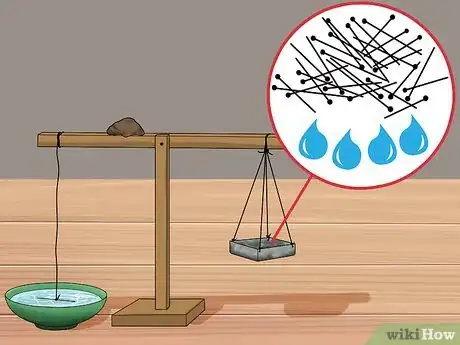
7Weigh a batch of pins or several measured drops of water on a small postal scale. You will be adding pins or drops of water one at a time to the aluminum dish you constructed earlier. For the calculation, it is important to know exactly how much weight is required to lift the needle out of the water.
- Count out a number of pins or drops of water and weigh them.
- Determine the individual weight of each drop or pin by dividing the total weight by the number of pins or water drops.
- For example, let’s say 30 pins weigh 15 grams: 15/30 = 0.5. Each pin weighs 0.5 grams.
-
8Add the pins or drops of water one at a time to your aluminum foil container until the needle is freed from the water's surface. Slowly add a pin or a drop of water to the aluminum dish one pin/drop at a time. Watch the needle closely to see if it comes out of the water with each new addition. Stop adding pins/drops once the needle is no longer in contact with the surface of the water.
- Count the number of pins or drops of water needed to remove the counterweight from the water's surface.
- Record each reading.
- Repeat the exercise several times (5 or 6) for more accurate readings.
- Calculate an average of the results by adding the total number of pins needed in each trial and dividing that by the total number of trials.
-
9Convert the measurement of pins into force by multiplying the number of grams by 0.00981 N/g. To calculate the surface tension, you need to know the total amount of force required to remove the needle from the surface of the liquid. Because you weighed the pins in the previous step, you can easily do this calculation using the conversion factor 0.00981 N/g.[5]
- Multiply the number of pins added to the dish by the weight of each pin. For example, 5 pins at 0.5 g/pin = 5 x 0.5 = 2.5 g.
- Multiply the amount of grams by the conversion factor 0.00981 N/g: 2.5 x 0.00981 = 0.025 N.
-
10Plug the variables into the equation and solve. Using the measurements you gathered throughout the experiment, you can now solve for force. Simply plug the numbers into the correct variable and solve using the proper order of operations.
- Continuing our example, let’s say the needle was 0.025 m long. Plugging the variables into the equation yields: S = F/2d = 0.025 N/(2 x 0.025) = 0.05 N/m. The surface tension of the liquid is 0.05 N/m.
Measuring Surface Tension with Capillary Action
-
1Understand capillary action. In order to understand capillary action, you first need to understand adhesion and cohesive forces. Adhesion is the force that causes a liquid to stick to a solid surface, such as the edge of a glass. Cohesive forces are those that pull liquid molecules toward each other.[6] The combination of adhesion and cohesive forces causes a liquid to rise up the center of a thin tube.
- The height the liquid rises can be used to calculate the surface tension of that liquid.
- Cohesion causes water to form bubbles or droplets on a surface. When a liquid is in contact with air, the molecules feel attractive forces towards each other and make a bubble on the surface.
- Adhesion causes the meniscus that is seen in liquids when they cling to the sides of a glass. It is the concave shape at the top of the liquid seen at eye level.[7]
- An example of capillary action is watching water rise in a straw placed in a cup of water.
-
2Define the equation to solve for surface tension. Surface tension is given by the equation S = (ρhga/2) where S is the surface tension, ρ (or rho) is the density of the liquid you are measuring, h is the height the liquid rises in the tube, g is the acceleration due to gravity acting on the liquid (9.8 m/s2) and a is the radius of the capillary tube.[8]
- When working through this equation, make sure all of your units are in the proper metric form: density in kg/m3, height and radius in meters, and gravity in m/s2.
- If the density of the liquid is not given, you can look it up in a reference book or calculate it using the equation density = mass/volume.
- The unit for surface tension is one newton per meter (N/m). A Newton is equal to 1 kg-m/s2. To work out the units on your own, simply solve the equation with just units. S = kg/m3 * m * m/s2 * m. Two of the meter units cancel out two of the per meter units and you are left with 1 kg-m/s2/m or 1 N/m.
-
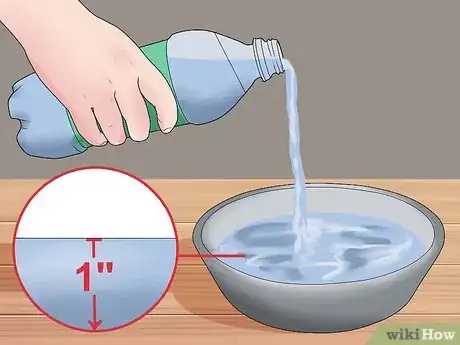
3Fill a container with the liquid of unknown surface tension. Using a shallow dish or bowl, fill it with about an inch of the liquid in question. The amount of liquid added does not matter as long as you can clearly see the rise in liquid within the capillary tube.[9]
- If you repeat this with different liquids, make sure the dish is thoroughly cleaned and dried before adding the next liquid. Alternatively, just use separate dishes for each liquid.
-
4Place a clear, thin tube into the liquid. This is the tube that you will take your measurements from to calculate the surface tension. The tube must be clear so that you can see how far the liquid rises above the level in the dish. The tube must also have the same radius throughout.[10]
- To measure the radius, simply place a ruler across the top of the tube and determine the diameter. Divide the diameter by 2 and you have the radius.
- You can buy these tubes online or from a hardware store.
- It can be difficult to accurately measure small changes in the height the liquid will rise in a straw or wide tube. As the height to which the water will rise is inversely proportional to the diameter of the tube (narrower tube = higher rise) this experiment is much easier to do with a narrow transparent capillary tube. These can be purchased at low cost online, but confirm the inside diameter is provided (typically around 1mm-1.2mm) and both ends are open. As these are fragile and made of glass, ensure care when handling them.
-
5Measure the height the liquid rises above the liquid in the container. Place the bottom of a ruler directly above the liquid in the dish and measure how high the liquid has risen into the tube. The water rises due to the upward force of surface tension being greater than the downward force of gravity.[11]
-
6Plug the measured values into the equation and solve. Once you have determined all of the necessary variables, you can plug them into the formula and solve for surface tension. Remember to convert all of your values to metric so the problem can be solved properly.
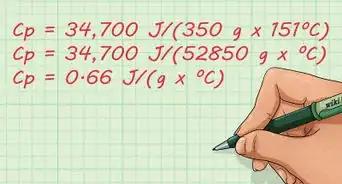
- For example, let’s say we are measuring the surface tension of water. Water has a density around 1000 kg/m3 (we will use approximate values in this example).[12] The variable g is always 9.8 m/s2. The radius of the tube is .029 m and the water rises 0.0005 m. What is the surface tension of the water?
- Plugging the variables into the equation yields: S = (ρhga/2) = (1000 x 9.8 x 0.029 x 0.0005)/2 = 0.1421/2 = 0.071 J/m2.
Measuring Relative Surface Tension with a Penny
-
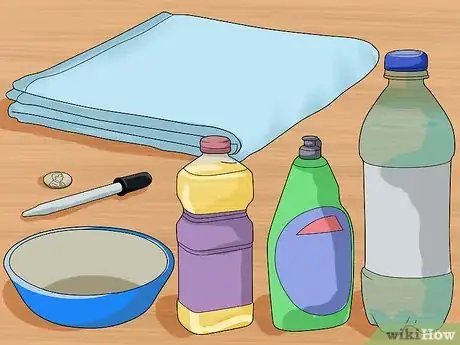
1Gather your materials. For this experiment you will need an eye dropper, a dry penny, water, a small bowl, dish soap, oil, and a towel. Most of these items can be found around the house or purchased at the grocery store. You don’t have to use dish soap and oil, but you will want different liquids to compare their surface tensions.[13]
- Make sure the penny is completely clean and dry before beginning the experiment. If there are other liquids on the penny, the experiment will not be accurate.
- This experiment does not allow you to calculate surface tension, but just determine surface tensions of different liquids relative to each other.
-
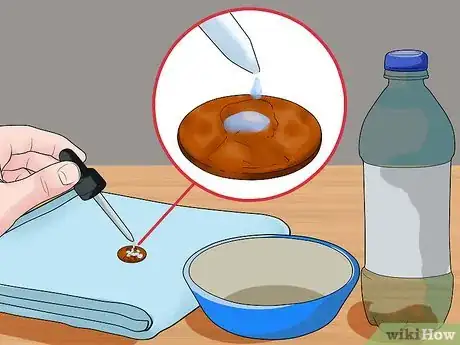
2Drip one drop of liquid at a time onto the penny. Place the penny on top of a towel or a surface that you don’t mind getting wet. Fill the eyedropper with the first liquid. Slowly, drip the liquid onto the penny, taking care to only drop one drop at a time. Count the number of drops it takes to fill the penny until the liquid runs over.[14]
- Write down how many drops it takes for the liquid to flow over the side of the penny.
-
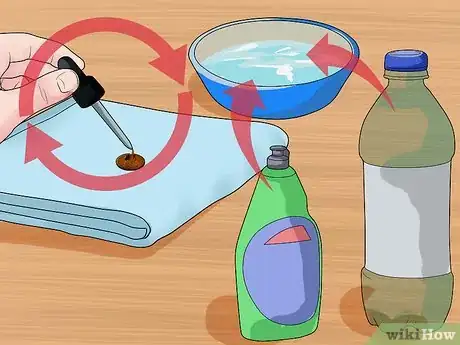
3Repeat the experiment with a different liquid. Clean and dry the penny in between each test of liquid. Dry the surface that you placed the penny on before repeating the experiment. Use multiple eyedroppers or clean it in between uses.[15]
- Try mixing a little bit of dish soap to the water and dropping again to see if the surface tension changes.
-
4Compare the number of drops it takes to fill the penny for each liquid. Try repeating the experiment with the same liquid multiple times to see if you are getting an accurate count. Average the trials together by adding them together and dividing by the number of trials performed. Write down which substances required the most drops and which required the fewest to fill the penny.[16]
- Substances with a higher surface tension will have more drops on the penny than substances with a lower surface tension.
- The dish soap lowers the surface tension of the water, using fewer drops to fill the penny.
Community Q&A
-
QuestionCan I use pennies instead of water droplets or pins?
 Community AnswerNo. Pennies have a very dense and heavy surface rather than water droplets or pins.
Community AnswerNo. Pennies have a very dense and heavy surface rather than water droplets or pins. -
QuestionI have a homemade, non-bulb pipette, 66 cm long. When shut, fluid does not escape. Is there a universal formula for this effect? Surface tension, adhesion, gravity, air pressure?
 Community AnswerIf the fluid in the pipette's exit flowed out, it would create a near vacuum, sucking the liquid back in. No gas can replace the liquid, so the only gas available attempts to fill the space. This creates the sucking force (like the principle of the vacuum cleaner) that keeps the liquid in place.
Community AnswerIf the fluid in the pipette's exit flowed out, it would create a near vacuum, sucking the liquid back in. No gas can replace the liquid, so the only gas available attempts to fill the space. This creates the sucking force (like the principle of the vacuum cleaner) that keeps the liquid in place. -
QuestionWhat do I do if method 2 is not working? I'm submerging the tube in water, but there is no liquid above where the liquid in the dish ends.
 Community AnswerUse a capillary tube that has a small inner radius. Moreover, use a travelling microscope for better readings and therefore better results.
Community AnswerUse a capillary tube that has a small inner radius. Moreover, use a travelling microscope for better readings and therefore better results.
Things You'll Need
- Straw, plastic ruler or other stiff rod
- String
- Aluminum foil
- Modeling clay or other similar material
- Long needle or nail for fulcrum
- Paper clip or needle to submerge into water
- Books or other material of equal weight to support the balance beam
- Calculator
- Small container
- Water
- Eye dropper, pipette or pins
- Postal scale or other small weighing device
- Shallow dish
References
- ↑ http://www.sciencebuddies.org/science-fair-projects/project_ideas/Chem_p021.shtml#background
- ↑ http://www.sciencebuddies.org/science-fair-projects/project_ideas/Phys_p012.shtml#background
- ↑ https://csef.usc.edu/History/2015/Projects/J1710.pdf
- ↑ http://www.sciencebuddies.org/science-fair-projects/project_ideas/Phys_p012.shtml#procedure
- ↑ http://www.sciencebuddies.org/science-fair-projects/project_ideas/Phys_p012.shtml#procedure
- ↑ https://www.teachengineering.org/view_lesson.php?url=collection/duk_/lessons/duk_surfacetensionunit_lessons/duk_surfacetensionunit_less2.xml
- ↑ https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/States_of_Matter/Properties_of_Liquids/Capillary_Action
- ↑ https://www.teachengineering.org/view_lesson.php?url=collection/duk_/lessons/duk_surfacetensionunit_lessons/duk_surfacetensionunit_less2.xml
- ↑ https://pressbooks.uiowa.edu/clonedbook/chapter/cohesion-and-adhesion-in-liquids-surface-tension-and-capillary-action/
- ↑ https://chem.libretexts.org/Courses/Oregon_Institute_of_Technology/OIT%3A_CHE_202_-_General_Chemistry_II/Unit_7%3A_Intermolecular_and_Intramolecular_Forces_in_Action/7.1%3A_Surface_Tension%2C_Viscosity%2C_and_Capillary_Action
- ↑ https://www.teachengineering.org/view_lesson.php?url=collection/duk_/lessons/duk_surfacetensionunit_lessons/duk_surfacetensionunit_less2.xml
- ↑ http://www.engineeringtoolbox.com/water-density-specific-weight-d_595.html
- ↑ https://www.scientificamerican.com/article/measure-surface-tension-with-a-penny/
- ↑ https://www.sciencebuddies.org/science-fair-projects/project-ideas/Chem_p021/chemistry/measuring-surface-tension-of-water-with-a-penny
- ↑ https://www.sciencebuddies.org/science-fair-projects/project-ideas/Chem_p021/chemistry/measuring-surface-tension-of-water-with-a-penny
- ↑ https://www.scientificamerican.com/article/measure-surface-tension-with-a-penny/
About This Article
To measure surface tension using the capillary method, fill a shallow dish with 1 inch of water. Measure the radius of a clear tube, then place the tube in the water and measure how high the water in the tube rises above the water in the container. Plug the measured value into your equation to calculate the surface tension. For more information on measuring surface tension from our Environmental Science reviewer, including how to calculate relative tension with a penny, keep reading.