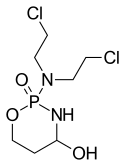
4-Hydroxycyclophosphamide
4-Hydroxycyclophosphamide is in the class of oxazaphosphorine compounds, and is the main, active metabolite of cyclophosphamide and of mafosfamide after they partially metabolized by cytochrome P450. It is then partially tautomerized into aldophosphamide, which, in turn, easily enters live cells and then is partially detoxified into inactive carboxycyclophosphamide by the enzyme ALDH, but partially is hydrolyzed by another cell's enzyme phosphatase to the two directly cytotoxic metabolites - phosphoramide mustard and acrolein.[1]
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C7H15Cl2N2O3P |
| Molar mass | 277.08 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
References
- Ludeman SM (August 1999). "The chemistry of the metabolites of cyclophosphamide". Current Pharmaceutical Design. 5 (8): 627–43. doi:10.2174/1381612805666230110215458. PMID 10469895.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.