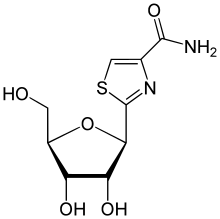
Tiazofurin
Tiazofurin is a drug which acts as an inhibitor of the enzyme IMP dehydrogenase. Tiazofurin and its analogues were under investigation for potential use in the treatment of cancer,[1] though side effects such as pleuropericarditis and a flu-like syndrome precluded further development. They also show antiviral effects and may be reevaluated as potential options in the treatment of newly emerging viral diseases.[2]
 | |
| Clinical data | |
|---|---|
| Other names | 2-[(2R,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3-thiazole-4-carboxamide |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C9H12N2O5S |
| Molar mass | 260.26 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
References
- Popsavin M, Torović L, Svircev M, et al. (2006). "Synthesis and antiproliferative activity of two new tiazofurin analogues with 2'-amido functionalities". Bioorg. Med. Chem. Lett. 16 (10): 2773–6. doi:10.1016/j.bmcl.2006.02.001. PMID 16495053.
- De Clercq E (March 2016). "C-Nucleosides To Be Revisited". Journal of Medicinal Chemistry. 59 (6): 2301–11. doi:10.1021/acs.jmedchem.5b01157. PMID 26513594.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.