Phospholipase
A phospholipase is an enzyme that hydrolyzes phospholipids[1] into fatty acids and other lipophilic substances. Acids trigger the release of bound calcium from cellular stores and the consequent increase in free cytosolic Ca2+, an essential step in calcium signaling to regulate intracellular processes.[2] There are four major classes, termed A, B, C, and D, which are distinguished by the type of reaction which they catalyze:
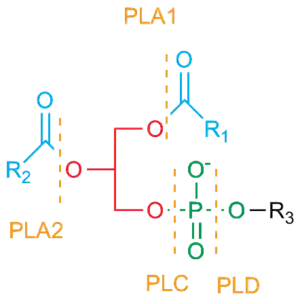
- Phospholipase A
- Phospholipase A1 – cleaves the sn-1 acyl chain (where sn refers to stereospecific numbering).
- Phospholipase A2 – cleaves the sn-2 acyl chain, releasing arachidonic acid.
- Phospholipase B – cleaves both sn-1 and sn-2 acyl chains; this enzyme is also known as a lysophospholipase.
- Phospholipase C – cleaves before the phosphate, releasing diacylglycerol and a phosphate-containing head group. PLCs play a central role in signal transduction, releasing the second messenger inositol triphosphate.
- Phospholipase D – cleaves after the phosphate, releasing phosphatidic acid and an alcohol.
Types C and D are considered phosphodiesterases.
Endothelial lipase is primarily a phospholipase.[3]
Phospholipase A2 acts on the intact lecithin molecule and hydrolyzes the fatty acid esterified to the second carbon atom. The resulting products are lysolecithin and a fatty acid. Phospholipase A2 is an enzyme present in the venom of bees, blennies and viper snakes.[4]
References
- "phospholipase" at Dorland's Medical Dictionary
- Molinari, Giuliano; Nervo, Elsa (2021-02-26). "Role of protons in calcium signaling". The Biochemical Journal. 478 (4): 895–910. doi:10.1042/BCJ20200971. ISSN 1470-8728. PMID 33635336.
- Yu JE, Han SY, Wolfson B, Zhou Q (2018). "The role of endothelial lipase in lipid metabolism, inflammation, and cancer". Histology and Histopathology. 33 (1): 1–10. doi:10.14670/HH-11-905. PMC 5858721. PMID 28540715.
- D. M. Vasudevan & S. Sreekumari, Textbook of Biochemistry (5th ed.)
Further reading
External links
- Phospholipases at the U.S. National Library of Medicine Medical Subject Headings (MeSH)