Baryte
Baryte, barite or barytes (/ˈbæraɪt, ˈbɛər-/[7] or /bəˈraɪtiːz/[8]) is a mineral consisting of barium sulfate (BaSO4).[3] Baryte is generally white or colorless, and is the main source of the element barium. The baryte group consists of baryte, celestine (strontium sulfate), anglesite (lead sulfate), and anhydrite (calcium sulfate). Baryte and celestine form a solid solution (Ba,Sr)SO4.[2]
| Baryte (barite) | |
|---|---|
 Baryte crystals from Cerro Huarihuyn, Miraflores, Huamalíes, Huánuco, Peru | |
| General | |
| Category | Sulfate mineral, barite group |
| Formula (repeating unit) | BaSO4 |
| IMA symbol | Brt[1] |
| Strunz classification | 7.AD.35 |
| Dana classification | 28.03.01.01 |
| Crystal system | Orthorhombic |
| Crystal class | Dipyramidal (mmm) H-M symbol: (2/m 2/m 2/m) |
| Space group | Pnma |
| Unit cell | a = 8.884(2) Å, b = 5.457(3) Å, c = 7.157(2) Å; Z = 4 |
| Identification | |
| Color | Colorless, white, light shades of blue, yellow, grey, brown |
| Crystal habit | Tabular parallel to base, fibrous, nodular to massive |
| Cleavage | Perfect cleavage parallel to base and prism faces: {001} Perfect, {210} Perfect, {010} Imperfect |
| Fracture | Irregular/uneven |
| Tenacity | Brittle |
| Mohs scale hardness | 3-3.5 |
| Luster | Vitreous, pearly |
| Streak | White |
| Diaphaneity | transparent to opaque |
| Specific gravity | 4.3–5 |
| Density | 4.48 g/cm3[2] |
| Optical properties | biaxial positive |
| Refractive index | nα = 1.634–1.637 nβ = 1.636–1.638 nγ = 1.646–1.648 |
| Birefringence | 0.012 |
| Fusibility | 4, yellowish green barium flame |
| Diagnostic features | white color, high specific gravity, characteristic cleavage and crystals |
| Solubility | low |
| References | [3][4][5][6] |
Names and history
The radiating form, sometimes referred to as Bologna Stone,[9] attained some notoriety among alchemists for specimens found in the 17th century near Bologna by Vincenzo Casciarolo. These became phosphorescent upon being calcined.[10][11]
Carl Scheele determined that baryte contained a new element in 1774, but could not isolate barium, only barium oxide. Johan Gottlieb Gahn also isolated barium oxide two years later in similar studies. Barium was first isolated by electrolysis of molten barium salts in 1808 by Sir Humphry Davy in England.[12]
The American Petroleum Institute specification API 13/ISO 13500, which governs baryte for drilling purposes, does not refer to any specific mineral, but rather a material that meets that specification.[13] In practice, however, this is usually the mineral baryte.[14]
The term "primary barytes" refers to the first marketable product, which includes crude baryte (run of mine) and the products of simple beneficiation methods, such as washing, jigging, heavy media separation, tabling, and flotation. Most crude baryte requires some upgrading to minimum purity or density. Baryte that is used as an aggregate in a "heavy" cement is crushed and screened to a uniform size. Most baryte is ground to a small, uniform size before it is used as a filler or extender, an addition to industrial products, in the production of barium chemicals or as a weighting agent in petroleum well drilling mud.[15]
Name
The name baryte is derived from the Ancient Greek: βαρύς, romanized: barús, 'heavy'. The American spelling is barite.[3][16] The International Mineralogical Association initially adopted "barite" as the official spelling, but recommended adopting the older "baryte" spelling later. This move was controversial and was notably ignored by American mineralogists.[17]
Other names have been used for baryte, including barytine,[18] barytite,[18] barytes,[19] heavy spar,[3] tiff,[4] and blanc fixe.[20]
Mineral associations and locations



Baryte occurs in many depositional environments, and is deposited through many processes including biogenic, hydrothermal, and evaporation, among others.[2] Baryte commonly occurs in lead-zinc veins in limestones, in hot spring deposits, and with hematite ore. It is often associated with the minerals anglesite and celestine. It has also been identified in meteorites.[21]
Baryte has been found at locations in Australia, Brazil, Nigeria, Canada, Chile, China, India, Pakistan, Germany, Greece, Guatemala, Iran, Ireland (where it was mined on Benbulben[22]), Liberia, Mexico, Morocco, Peru, Romania (Baia Sprie), Turkey, South Africa (Barberton Mountain Land),[23] Thailand, United Kingdom (Cornwall, Cumbria, Dartmoor/Devon, Derbyshire, Durham, Shropshire,[24] Perthshire, Argyllshire, and Surrey[3]) and in the US from Cheshire, Connecticut, De Kalb, New York, and Fort Wallace, New Mexico. It is mined in Arkansas, Connecticut, Virginia, North Carolina, Georgia, Tennessee, Kentucky, Nevada, and Missouri.[3]
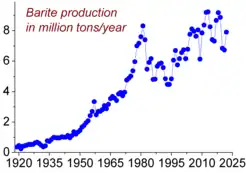
The global production of baryte in 2019 was estimated to be around 9.5 million metric tons, down from 9.8 million metric tons in 2012.[25] The major barytes producers (in thousand tonnes, data for 2017) are as follows: China (3,600), India (1,600), Morocco (1,000), Mexico (400), United States (330), Iran (280), Turkey (250), Russia (210), Kazakhstan (160), Thailand (130) and Laos (120).[26]
The main users of barytes in 2017 were (in million tonnes) US (2.35), China (1.60), Middle East (1.55), the European Union and Norway (0.60), Russia and CIS (0.5), South America (0.35), Africa (0.25), and Canada (0.20). 70% of barytes was destined for oil and gas well drilling muds. 15% for barium chemicals, 14% for filler applications in automotive, construction, and paint industries, and 1% other applications.[26]
Natural baryte formed under hydrothermal conditions may be associated with quartz or silica.[27] In hydrothermal vents, the baryte-silica mineralisation can also be accompanied by precious metals.[28]
Uses
In oil and gas drilling
Worldwide, 69–77% of baryte is used as a weighting agent for drilling fluids in oil and gas exploration to suppress high formation pressures and prevent blowouts. As a well is drilled, the bit passes through various formations, each with different characteristics. The deeper the hole, the more baryte is needed as a percentage of the total mud mix. An additional benefit of baryte is that it is non-magnetic and thus does not interfere with magnetic measurements taken in the borehole, either during logging-while-drilling or in separate drill hole logging. Baryte used for drilling petroleum wells can be black, blue, brown or gray depending on the ore body. The baryte is finely ground so that at least 97% of the material, by weight, can pass through a 200-mesh (75 μm) screen, and no more than 30%, by weight, can be less than 6 μm diameter. The ground baryte also must be dense enough so that its specific gravity is 4.2 or greater, soft enough to not damage the bearings of a tricone drill bit, chemically inert, and containing no more than 250 milligrams per kilogram of soluble alkaline salts.[16] In August 2010, the American Petroleum Institute published specifications to modify the 4.2 drilling grade standards for baryte to include 4.1 SG materials.
In oxygen and sulfur isotopic analysis

In the deep ocean, away from continental sources of sediment, pelagic baryte precipitates and forms a significant amount of the sediments. Since baryte has oxygen, systematics in the δ18O of these sediments have been used to help constrain paleotemperatures for oceanic crust.
The variations in sulfur isotopes (34S/32S) are being examined in evaporite minerals containing sulfur (e.g. baryte) and carbonate associated sulfates (CAS) to determine past seawater sulfur concentrations which can help identify specific depositional periods such as anoxic or oxic conditions. The use of sulfur isotope reconstruction is often paired with oxygen when a molecule contains both elements.[29]
Geochronological dating
Dating the baryte in hydrothermal vents has been one of the major methods to know the ages of hydrothermal vents.[30][31][32][33][34] Common methods to date hydrothermal baryte include radiometric dating[30][31] and electron spin resonance dating.[32][33][34]
Other uses
Baryte is used in added-value applications which include filler in paint and plastics, sound reduction in engine compartments, coat of automobile finishes for smoothness and corrosion resistance, friction products for automobiles and trucks, radiation shielding concrete, glass ceramics, and medical applications (for example, a barium meal before a contrast CT scan). Baryte is supplied in a variety of forms and the price depends on the amount of processing; filler applications commanding higher prices following intense physical processing by grinding and micronising, and there are further premiums for whiteness and brightness and color.[16] It is also used to produce other barium chemicals, notably barium carbonate which is used for the manufacture of LED glass for television and computer screens (historically in cathode ray tubes); and for dielectrics.
Historically, baryte was used for the production of barium hydroxide for sugar refining, and as a white pigment for textiles, paper, and paint.[3]
Although baryte contains the toxic alkaline earth metal barium, it is not detrimental for human health, animals, plants and the environment because barium sulfate is extremely insoluble in water.
See also
References
- Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- Hanor, J. (2000). "Barite-celestine geochemistry and environments of formation". Reviews in Mineralogy. Washington, DC: Mineralogical Society of America. 40 (1): 193–275. Bibcode:2000RvMG...40..193H. doi:10.2138/rmg.2000.40.4. ISBN 0-939950-52-9.
- Dana, James Dwight; Ford, William Ebenezer (1915). Dana's Manual of Mineralogy for the Student of Elementary Mineralogy, the Mining Engineer, the Geologist, the Prospector, the Collector, Etc (13 ed.). John Wiley & Sons, Inc. pp. 299–300.
- Barite at Mindat
- Webmineral data for barite
- Baryte, Handbook of Mineralogy
- "baryte". Lexico UK English Dictionary. Oxford University Press. Archived from the original on March 8, 2020.
- "barytes". Merriam-Webster.com Dictionary.
- Jackson, Julia A., ed. (1997). "Bologna stone". Glossary of geology (Fourth ed.). Alexandria, Virginia: American Geological Institute. ISBN 0922152349.
- History of the Bologna stone Archived 2006-12-02 at the Wayback Machine
- Lastusaari, Mika; Laamanen, Taneli; Malkamäki, Marja; Eskola, Kari O.; Kotlov, Aleksei; Carlson, Stefan; Welter, Edmund; Brito, Hermi F.; Bettinelli, Marco; Jungner, Högne; Hölsä, Jorma (26 September 2012). "The Bologna Stone: history's first persistent luminescent material" (PDF). European Journal of Mineralogy. 24 (5): 885–890. Bibcode:2012EJMin..24..885L. doi:10.1127/0935-1221/2012/0024-2224. S2CID 97905966.
- Krebs, Robert E. (2006). The history and use of our earth's chemical elements: a reference guide. Greenwood Publishing Group. p. 80. ISBN 978-0-313-33438-2.
- "ISO 13500:2008 Petroleum and natural gas industries — Drilling fluid materials — Specifications and tests". ISO. 2008. Retrieved 2 February 2022.
- Nesse, William D. (2000). Introduction to mineralogy. New York: Oxford University Press. pp. 345–346. ISBN 9780195106916.
-
 This article incorporates text from a free content work. Licensed under Public domain. Text taken from Barite Statistics and Information, National Minerals Information Center, U.S. Geological Survey.
This article incorporates text from a free content work. Licensed under Public domain. Text taken from Barite Statistics and Information, National Minerals Information Center, U.S. Geological Survey. - M. Michael Miller Barite, 2009 Minerals Yearbook
- "Barite: The mineral Barite information and pictures". www.minerals.net. Retrieved 2017-12-14.
- "International Mineralogical Association: Commission on New Minerals and Mineral Names". Mineralogical Magazine. 38 (293): 102–5. March 1971. Bibcode:1971MinM...38..102.. doi:10.1180/minmag.1971.038.293.14.
- "Monograph on Barytes". Indian Bureau of Mines. 1995. Retrieved 14 July 2017.
- "Definition of blanc fixe". Merriam-Webster Dictionary. Merriam-Webster. Retrieved 14 July 2017.
- Rubin, Alan E. (March 1997). "Mineralogy of meteorite groups". Meteoritics & Planetary Science. 32 (2): 231–247. Bibcode:1997M&PS...32..231R. doi:10.1111/j.1945-5100.1997.tb01262.x.
- Ben Bulben. Mhti.com. Retrieved on 2011-05-05.
- Duchač, K. C; Hanor, J. S. (September 1987). "Origin and timing of the metasomatic silicification of an early Archaean komatiite sequence, Barberton Mountain Land, South Africa". Precambrian Research. 37 (2): 125–146. Bibcode:1987PreR...37..125D. doi:10.1016/0301-9268(87)90075-1.
- Muirshiel Mine. Clyde Muirshiel Regional Park. Scotland.
- "Production of barite worldwide 2019". Statista. Retrieved 2020-08-30.
- "The Barytes Association, Barytes Statistics". Archived from the original on 2015-05-18. Retrieved 2015-05-11.
- Fedele, L.; Todesca, R.; Boni, M. (2003). "Barite-silica mineralization at the inter-Ordovician unconformity in southwestern Sardinia (Italy): a fluid inclusion study". Mineralogy and Petrology. 77 (3–4): 197–213. Bibcode:2003MinPe..77..197F. doi:10.1007/s00710-002-0200-9. S2CID 129874363.
- Binns, R.A.; Parr, J.M.; Gemmell, J.B.; Whitford, D.J.; Dean, J.A. (1997). "Precious metals in barite-silica chimneys from Franklin Seamount, Woodlark Basin, Papua New Guinea". Marine Geology. 142 (1–4): 119–141. Bibcode:1997MGeol.142..119B. doi:10.1016/S0025-3227(97)00047-9.
- Kastner, Miriam (30 March 1999). "Oceanic minerals: Their origin, nature of their environment, and significance". Proc. Natl. Acad. Sci. U.S.A. 96 (7): 3380–7. Bibcode:1999PNAS...96.3380K. doi:10.1073/pnas.96.7.3380. PMC 34278. PMID 10097047.
- Grasty, Robert L.; Smith, Charles; Franklin, James M.; Jonasson, Ian R. (1988-09-01). "Radioactive orphans in barite-rich chimneys, Axial Caldera, Juan De Fuca Ridge". The Canadian Mineralogist. 26 (3): 627–636.
- Noguchi, Takuroh; Shinjo, Ryuichi; Ito, Michihiro; Takada, Jitsuya; Oomori, Tamotsu (2011). "Barite geochemistry from hydrothermal chimneys of the Okinawa Trough: insight into chimney formation and fluid/sediment interaction". Journal of Mineralogical and Petrological Sciences. 106 (1): 26–35. Bibcode:2011JMPeS.106...26N. doi:10.2465/jmps.090825.
- Takamasa, Asako; Nakai, Shun'ichi; Sato, Fumihiro; Toyoda, Shin; Banerjee, Debabrata; Ishibashi, Junichiro (February 2013). "U–Th radioactive disequilibrium and ESR dating of a barite-containing sulfide crust from South Mariana Trough". Quaternary Geochronology. 15: 38–46. doi:10.1016/j.quageo.2012.12.002. S2CID 129020357.
- Fujiwara, Taisei; Toyoda, Shin; Uchida, Ai; Ishibashi, Jun-ichiro; Nakai, Shun’ichi; Takamasa, Asako (2015), Ishibashi, Jun-ichiro; Okino, Kyoko; Sunamura, Michinari (eds.), "ESR Dating of Barite in Sea-Floor Hydrothermal Sulfide Deposits in the Okinawa Trough", Subseafloor Biosphere Linked to Hydrothermal Systems, Tokyo: Springer Japan, pp. 369–386, doi:10.1007/978-4-431-54865-2_29, ISBN 978-4-431-54864-5
- Tsang, Man-Yin; Toyoda, Shin; Tomita, Makiko; Yamamoto, Yuzuru (2022-08-01). "Thermal stability and closure temperature of barite for electron spin resonance dating". Quaternary Geochronology. 71: 101332. doi:10.1016/j.quageo.2022.101332. S2CID 248614826.
- Thomas, Arthur (2009). Gemstones: Properties, identification and use. New Holland Publishers. p. 138. ISBN 1847734847
Further reading
- Johnson, Craig A.; Piatak, Nadine M.; Miller, M. Michael; Schulz, Klaus J.; DeYoung, John H.; Seal, Robert R.; Bradley, Dwight C. (2017). "Barite (Barium). Chapter D of: Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply. Professional Paper 1802–D". U.S. Geological Survey Professional Papers. doi:10.3133/pp1802D.
![]() This article incorporates public domain material from Barite (PDF). United States Geological Survey.
This article incorporates public domain material from Barite (PDF). United States Geological Survey.