Brodimoprim
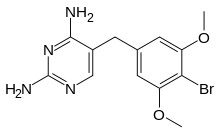
Brodimoprim is a structural derivative of trimethoprim. In brodimoprim, the 4-methoxy group of trimethoprim is replaced with a bromine atom.
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.054.745 |
| Chemical and physical data | |
| Formula | C13H15BrN4O2 |
| Molar mass | 339.193 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 225 to 228 °C (437 to 442 °F) |
| |
| |
| (verify) | |
As trimethoprim, brodimoprim is a selective inhibitor of bacterial dihydrofolate reductase.[1]
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.