Bucillamine
Bucillamine is an antirheumatic agent developed from tiopronin. Activity is mediated by the two thiol groups that the molecule contains. Research done in USA showed positive transplant preservation properties.[1] Bucillamine is currently being investigated for COVID-19 drug repurposing.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
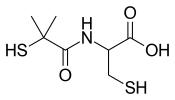
2-[(2-Methyl-2-sulfanylpropanoyl)amino]-3-sulfanylpropanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| MeSH | bucillamine |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H13NO3S2 | |
| Molar mass | 223.31 g·mol−1 |
| log P | 1.032 |
| Acidity (pKa) | 3.012 |
| Basicity (pKb) | 10.985 |
| Pharmacology | |
| M01CC02 (WHO) | |
| Related compounds | |
Related Alkanoic acids |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Bucillamine has a well-known safety profile and is prescribed in the treatment of rheumatoid arthritis in Japan and South Korea for over 30 years. It is a cysteine derivative with 2 thiol groups that is 16-fold more potent than acetylcysteine (NAC) as a thiol donor in vivo, giving it vastly superior function in restoring glutathione and therefore greater potential to prevent acute lung injury during influenza infection.[2] Bucillamine has also been shown to prevent oxidative and reperfusion injury in heart and liver tissues.[2]
Bucillamine has both proven safety and proven mechanism of action similar to that of NAC, but with much higher potency, mitigating the previous obstacles to using thiols therapeutically. It is hypothesized that similar processes related to reactive oxygen species (ROS) are involved in acute lung injury during nCov-19 infection, possibly justifying the investigation of bucillamine as an intervention for COVID-19.[3]
On July 31, 2020, the U.S. Food & Drug Administration (FDA) has approved Revive Therapeutics Ltd. to proceed with a randomized, double-blind, placebo-controlled confirmatory Phase 3 clinical trial protocol to evaluate the safety and efficacy of Bucillamine in patients with mild-moderate COVID-19.[4]
References
- Amersi, F.; Nelson, S. K.; Shen, X. D.; Kato, H.; Melinek, J.; Kupiec-Weglinski, J. W.; Horwitz, L. D.; Busuttil, R. W.; Horwitz, M. A. (2002). "Bucillamine, a Thiol Antioxidant, Prevents Transplantation-Associated Reperfusion Injury". Proceedings of the National Academy of Sciences. 99 (13): 8915–8920. Bibcode:2002PNAS...99.8915A. doi:10.1073/pnas.132026099. PMC 124398. PMID 12084933.
- Horwitz, Lawrence D. (2003). "Bucillamine: a potent thiol donor with multiple clinical applications". Cardiovascular Drug Reviews. 21 (2): 77–90. doi:10.1111/j.1527-3466.2003.tb00107.x. ISSN 0897-5957. PMID 12847560.
- "INFECTIOUS DISEASES | Revive Therapeutics". Retrieved 2020-08-14.
- Ltd, Revive Therapeutics (2020-07-31). "UPDATE - Revive Therapeutics Announces U.S. FDA Approval of Confirmatory Phase 3 Clinical Trial for Bucillamine in COVID-19". GlobeNewswire News Room. Retrieved 2020-08-05.